Label: LUCEMYRA- lofexidine hydrochloride tablet, film coated
- NDC Code(s): 78670-050-03, 78670-050-36, 78670-050-96
- Packager: USWM, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUCEMYRA safely and effectively. See full prescribing information for LUCEMYRA. LUCEMYRA® (lofexidine) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELUCEMYRA is indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The usual LUCEMYRA starting dosage is three 0.18 mg tablets taken orally 4 times daily during the period of peak withdrawal symptoms (generally the first 5 to 7 days ...
-
3 DOSAGE FORMS AND STRENGTHSLUCEMYRA is available as round, peach-colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side. Each tablet contains 0.18 mg lofexidine (equivalent to 0.2 mg of ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Hypotension, Bradycardia, and Syncope - LUCEMYRA can cause a decrease in blood pressure, a decrease in pulse, and syncope [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)] ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in labeling: Hypotension, Bradycardia, and Syncope [see Warnings and Precautions (5.1)] QT Prolongation [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Methadone - LUCEMYRA and methadone both prolong the QT interval. ECG monitoring is recommended in patients receiving methadone and LUCEMYRA concomitantly [see Warnings and Precautions (5.2) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The safety of LUCEMYRA in pregnant women has not been established. In animal reproduction studies, oral administration of lofexidine during organogenesis to ...
-
10 OVERDOSAGEOverdose with LUCEMYRA may manifest as hypotension, bradycardia, and sedation. In the event of acute overdose, perform gastric lavage where appropriate. Dialysis will not remove a substantial ...
-
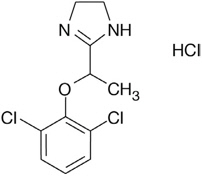
11 DESCRIPTIONLUCEMYRA tablets contain lofexidine, a central alpha-2 adrenergic agonist, as the hydrochloride salt. Lofexidine hydrochloride is chemically designated as 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No adequate long-term animal studies have been completed to evaluate the carcinogenic potential of ...
-
14 CLINICAL STUDIESTwo randomized, double-blind, placebo-controlled trials supported the efficacy of LUCEMYRA. Study 1, NCT01863186 - Study 1 was a 2-part efficacy, safety, and dose-response study conducted in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Available as 0.18 mg round, convex-shaped, peach colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side; approximately 7 mm in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). LUCEMYRA may mitigate, but not completely prevent, the symptoms associated with opioid withdrawal syndrome, which ...
-
SPL UNCLASSIFIED SECTIONThis product's Prescribing Information may have been updated. For current full Prescribing Information, please visit www.usworldmeds.com. Distributed by: USWM, LLC 4441 Springdale Road ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. 360-10020.01Issued: 09/2020 - PATIENT INFORMATION - LUCEMYRA® (LEW-sem-EER-uh) (lofexidine ...
-
PRINCIPAL DISPLAY PANEL - 0.18 mg Tablet Bottle CartonRx only - NDC 78670-050-96 - Lucemyra® (lofexidine) tablets - 0.18 mg - Store and dispense - in original container. Protect from heat and moisture. Do not remove desiccants. Keep the bottle tightly ...
-
INGREDIENTS AND APPEARANCEProduct Information