Label: LO SIMPESSE- levonorgestrel and ethinyl estradiol and ethinyl estradiol kit
- NDC Code(s): 65862-866-94, 65862-866-95
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LO SIMPESSE safely and effectively. See full prescribing information for LO SIMPESSE. LO SIMPESSETM (levonorgestrel and ethinyl ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptives (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including Lo Simpesse, are contraindicated in women who are over 35 years of age and smoke [see Contraindications (4) and Warnings and Precautions (5.1)].
Close -
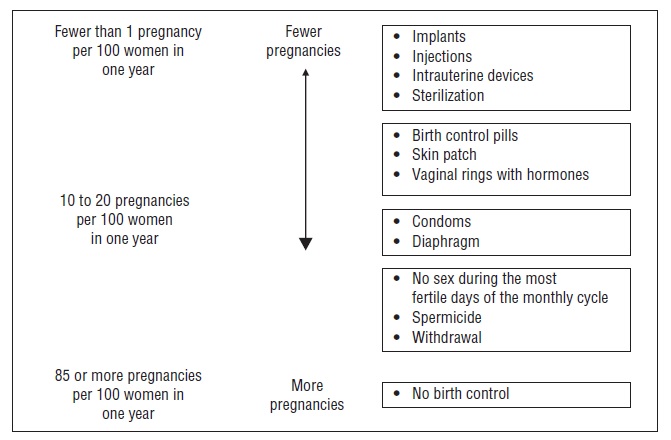
1 INDICATIONS AND USAGELo SimpesseTM (Levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets) is indicated for use by females of reproductive potential to prevent pregnancy.
-
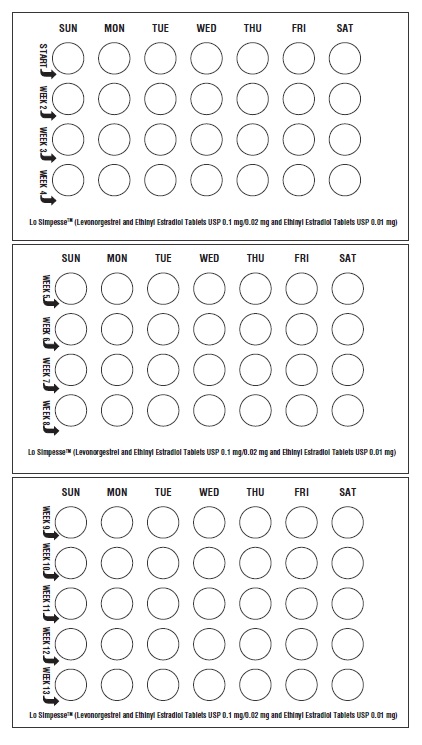
2 DOSAGE AND ADMINISTRATION2.1 How to Start and Take Lo Simpesse - Begin Lo Simpesse on the first Sunday after the onset of menstruation. If menstruation begins on a Sunday, take the first light pink tablet that day. For ...
-
3 DOSAGE FORMS AND STRENGTHSLo Simpesse is available in Extended-Cycle Wallets, each containing a 13-week supply of tablets: 84 light pink tablets, each containing 0.1 mg of levonorgestrel and 0.02 mg ethinyl estradiol, and ...
-
4 CONTRAINDICATIONSLo Simpesse is contraindicated in females who are known to have or develop the following conditions: A high risk of arterial or venous thrombotic diseases. Examples include females who are ...
-
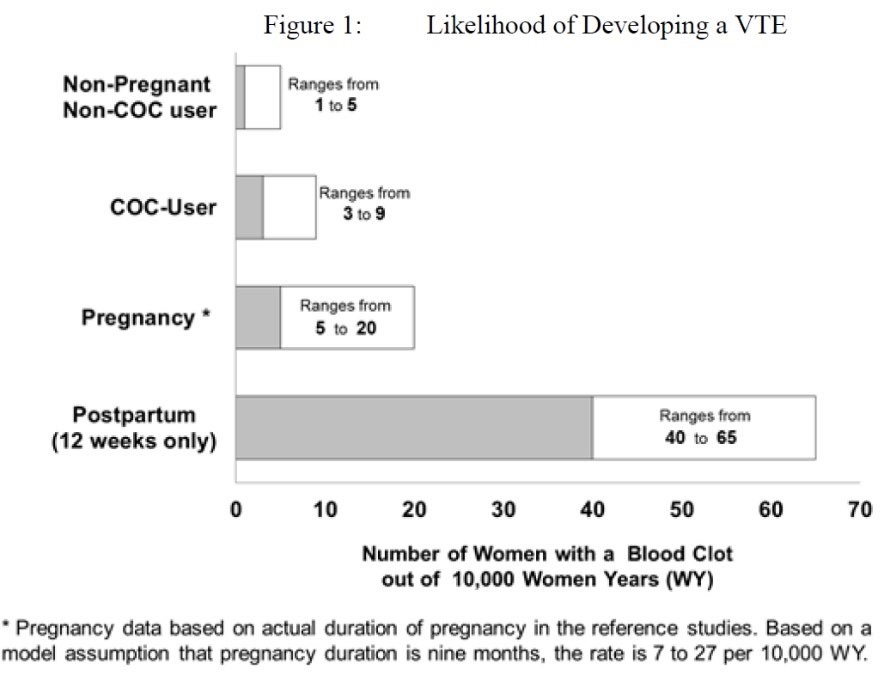
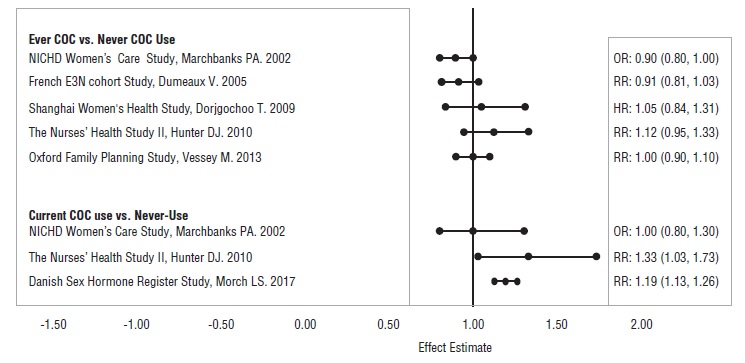
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolic Disorders and Other Vascular Conditions - Stop Lo Simpesse if an arterial or deep venous thrombotic/thromboembolic event occurs. Stop Lo Simpesse if there is unexplained loss ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling: Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions ...
-
7 DRUG INTERACTIONSThe sections below provide information on substances for which data on drug interactions with COCs are available. There is little information available about the clinical effect of most drug ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There is no use for contraception in pregnancy; therefore, Lo Simpesse should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not ...
-
10 OVERDOSAGEThere have been no reports of serious ill effects from overdose of oral contraceptives, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
-
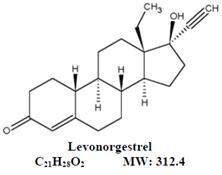
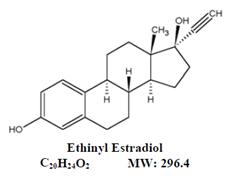
11 DESCRIPTIONLo Simpesse (levonorgestrel and ethinyl estradiol tablets USP and ethinyl estradiol tablets USP) is an extended-cycle oral contraceptive regimen of 84 light pink tablets each containing 0.1 mg ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - COCs prevent pregnancy primarily by suppressing ovulation. 12.2 Pharmacodynamics - No pharmacodynamic studies were conducted with Lo Simpesse. 12.3 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - [See Warnings and Precautions (5.2, 5.11)]
-
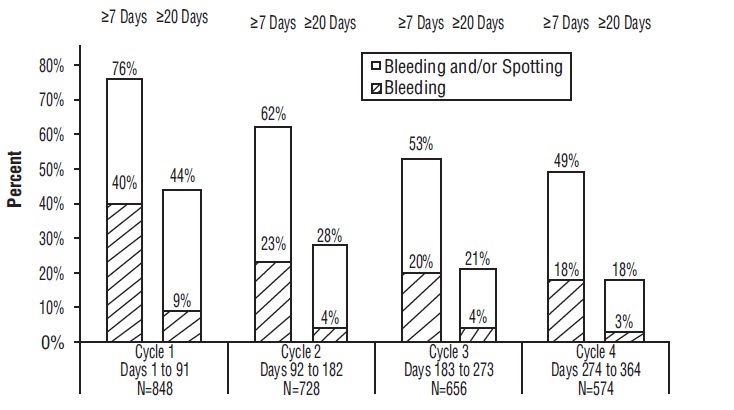
14 CLINICAL STUDIESIn a 12-month multicenter open-label clinical trial, 2,185 women aged 18 to 41 were studied to assess the safety and efficacy of Lo Simpesse, completing the equivalent of 20,937 28-day cycles of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Lo Simpesse (Levonorgestrel and Ethinyl Estradiol Tablets USP 0.1 mg/0.02 mg and Ethinyl Estradiol Tablets USP 0.01 mg) are available in an Extended-Cycle Wallets, that contains 84 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Counsel patients about the following information: Cigarette Smoking - Cigarette ...
-
FDA-approved Patient LabelingPATIENT INFORMATION - Lo SimpesseTM (loe sim pe' see) (Levonorgestrel and Ethinyl Estradiol Tablets USP 0.1 mg/0.02 mg and Ethinyl Estradiol Tablets USP 0.01 mg) WARNING TO WOMEN WHO ...
-
INSTRUCTIONS FOR USELo Simpesse (loe sim pe' see) (Levonorgestrel and Ethinyl Estradiol Tablets USP 0.1 mg/0.02 mg and Ethinyl - Estradiol Tablets USP 0.01 mg) How do I take Lo Simpesse? Take one pill every ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.1 mg/0.02 mg and 0.01 mg (91 Tablets Pouch)NDC 65862-866-94 - Lo SimpesseTM - (Levonorgestrel and Ethinyl Estradiol - Tablets USP 0.1 mg/0.02 mg and - Ethinyl Estradiol Tablets USP 0.01 mg) Rx ...
-
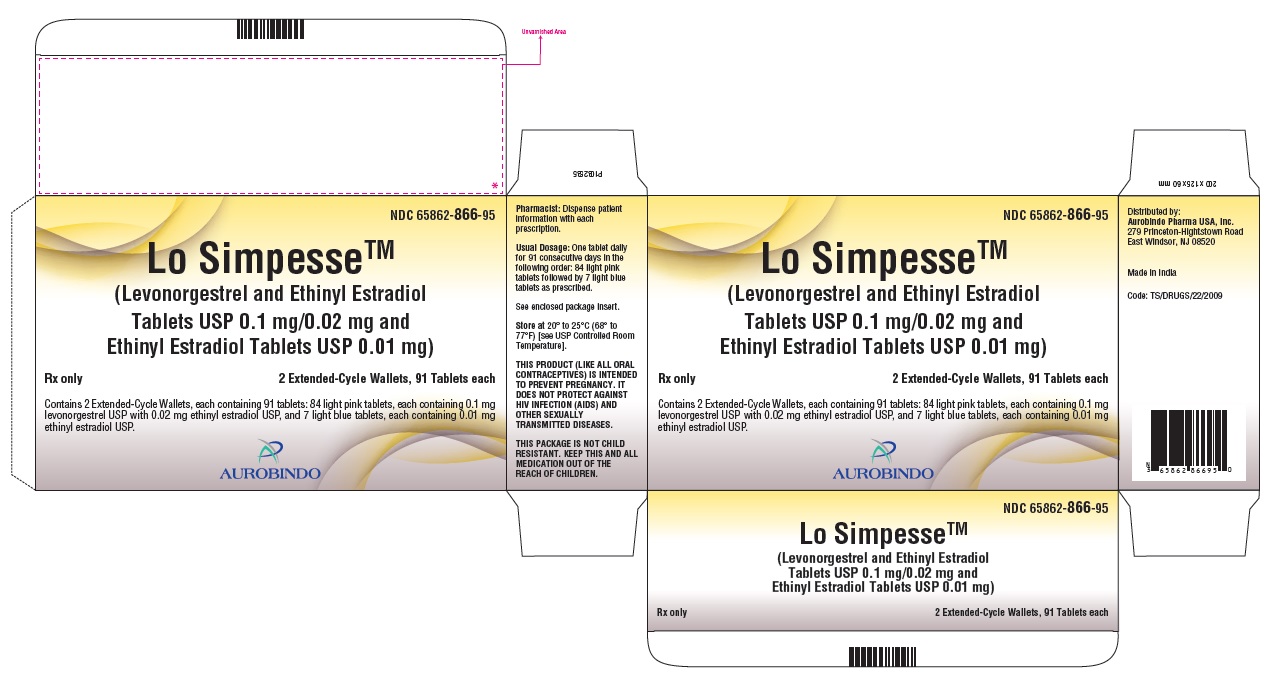
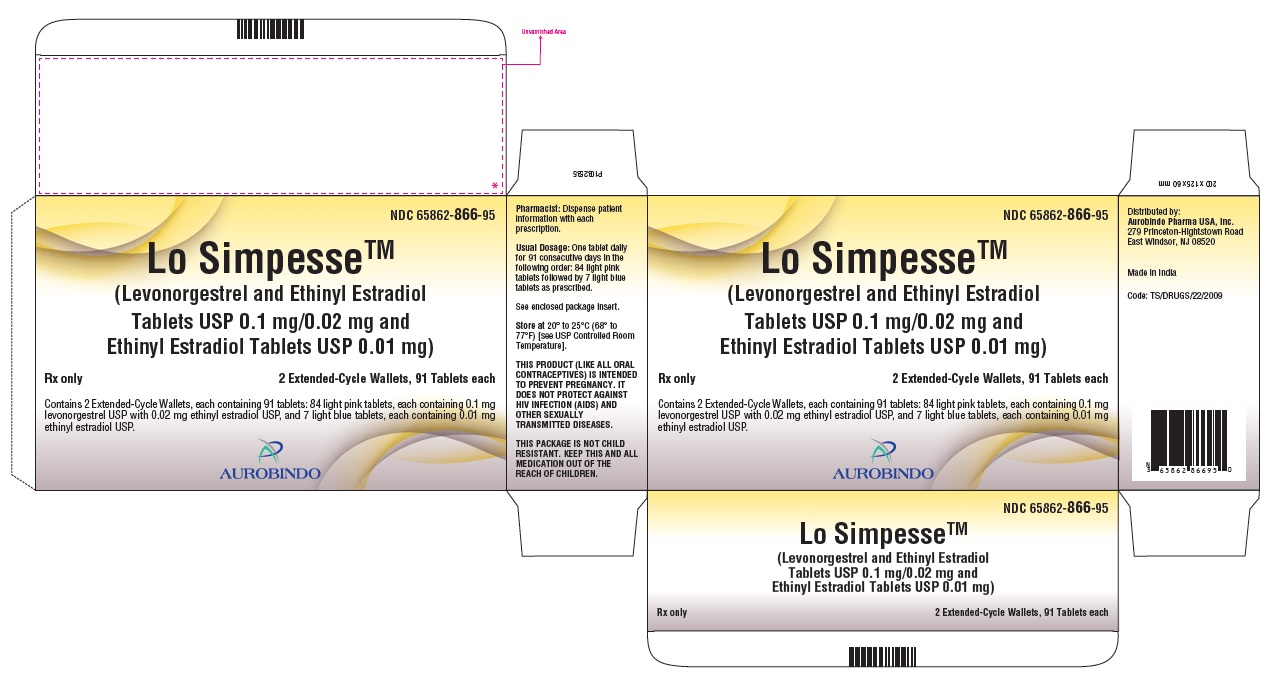
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.1 mg/0.02 mg and 0.01 mg (91 Tablets Carton)NDC 65862-866-95 - Lo SimpesseTM - (Levonorgestrel and Ethinyl Estradiol - Tablets USP 0.1 mg/0.02 mg and - Ethinyl Estradiol Tablets USP 0.01 mg) Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information