Label: LIDOXRYL- lidocaine kit
- NDC Code(s): 59088-396-54, 59088-722-00
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 10, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
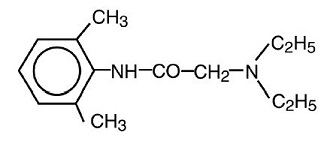
DESCRIPTION
Lidocaine patch 5% is comprised of an adhesive material containing 5% lidocaine, which is applied to a white non-woven polyethylene terephthalate (PET) material backing and covered with a ...
-
CLINICAL PHARMACOLOGY
Pharmacodynamics - Lidocaine is an amide-type local anesthetic agent and is suggested to stabilize neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction ...
-
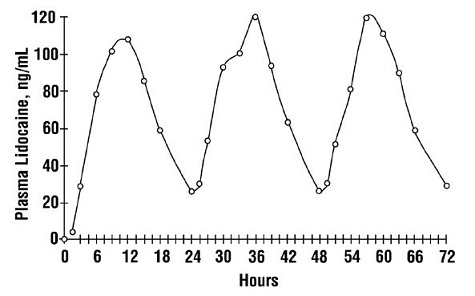
CLINICAL STUDIES
Single-dose treatment with lidocaine patch was compared to treatment with vehicle patch (without lidocaine), and to no treatment (observation only) in a double-blind, crossover clinical trial with ...
-
INDICATION AND USAGE
Lidocaine patch 5% is indicated for relief of pain associated with post-herpetic neuralgia. It should be applied only to - intact skin.
-
CONTRAINDICATIONS
Lidocaine patch 5% is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type, or to any other component of the product.
-
WARNINGS
Accidental Exposure in Children - Even a - used lidocaine patch contains a large amount of lidocaine (at least 665 mg). The potential exists for a small child or a pet to suffer ...
-
PRECAUTIONS
General - Hepatic Disease: Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of lidocaine, because of their inability to metabolize lidocaine ...
-
ADVERSE REACTIONS
Application Site Reactions - During or immediately after treatment with lidocaine patch 5%, the skin at the site of application may develop blisters, bruising, burning sensation ...
-
OVERDOSAGE
Lidocaine overdose from cutaneous absorption is rare, but could occur. If there is any suspicion of lidocaine overdose (see - ADVERSE REACTIONS, Systemic Reactions), drug ...
-
DOSAGE AND ADMINISTRATION
Apply lidocaine patch 5% to intact skin to cover the most painful area. Apply the prescribed number of patches (maximum of 3), only once for up to 12 hours within a 24-hour period. Patches may be ...
-
HANDLING AND DISPOSAL
Hands should be washed after the handling of lidocaine patch 5%, and eye contact with lidocaine patch 5% should be avoided. Do not store patch outside the sealed envelope. Apply immediately after ...
-
HOW SUPPLIED
Lidocaine patch 5% is available as the following: Carton of 15 patches, NDC 59088-396-82 - Carton of 30 patches, NDC 59088-396-54 - Each patch is packaged into an individual ...
-
Xrylix™ Sheets (Kinesiology Tape / Cross Tape)Xrylix™ Sheets is a taping therapy based on acupuncture principles to improve circulation through the skin and control tension and relief of the muscles and the ligaments. The tape is ...
-
SPL UNCLASSIFIED SECTIONDirections for use: Use only as prescribed by your physician. Apply to clean, dry skin only. Apply tape to affected area as directed, then rub the tape to activate the ...
-
SPL UNCLASSIFIED SECTIONGeneral taping for most body parts - Apply tape where you have strain, sprains, muscle stiffness, inflammation or pain.
-
SPL UNCLASSIFIED SECTIONTaping for the knee and feet - Knee(s): Apply tape to the knee as directed. Ankle(s): Apply tape to the ankle as directed. Feet: Apply tape on the center of the dorsum of ...
-
SPL UNCLASSIFIED SECTIONPrecautions - Use only as directed by your physician. Remove tape and discontinue use if tape causes itching or trash. Do not use on the wounds, skin infections or other parts where there is a ...
-
SPL UNCLASSIFIED SECTIONKeep out of direct sunlight and Under 40°C (104 °F). Storage time above these conditions should not exceed 18 hours. Manufactured for: PureTek Corporation - San ...
-
Lidoxryl

-
INGREDIENTS AND APPEARANCEProduct Information