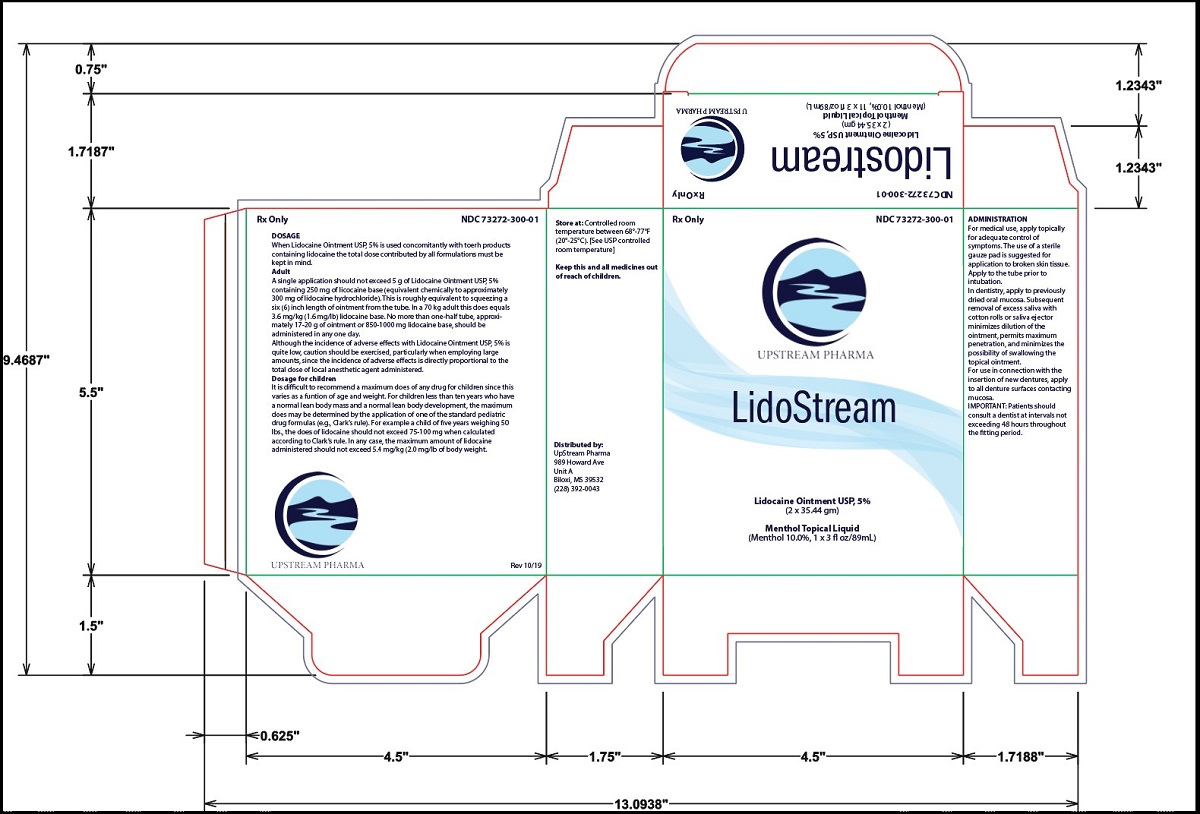
Label: LIDOSTREAM- lidocaine 5% ointment and menthol 10% topical liquid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 73272-300-01 - Packager: Upstream Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
-
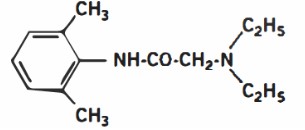
DescriptionLidocaine Ointment USP 5% contains a local anesthetic agent and is administered topically. See INDICATIONS AND USAGE for specific uses. Lidocaine Ointment USP 5% contains lidocaine, which is ...
-
Clinical PharmacologyMechanism of action - Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
Indications and UsageLidocaine Ointment USP, 5% is indicated for production of anesthesia of accessible mucous membranes of the oropharynx. It is also useful as an anesthetic lubricant for intubation and for the ...
-
ContraindicationsLidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components of Lidocaine Ointment USP, 5%.
-
WarningsEXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS, PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ...
-
PrecautionsGeneral - The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. (See WARNINGS and ADVERSE REACTIONS). The lowest ...
-
Adverse ReactionsAdverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general ...
-
OverdosageAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics. (see ADVERSE REACTIONS, WARNINGS, and ...
-
Dosage and AdministrationWhen Lidocaine Ointment USP, 5% is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind. Adult - A single application ...
-
How SuppliedLidocaine Ointment USP, 5% is available in 35.44 g (1 ¼ oz) laminate tubes with a child-resistant cap, NDC 52565-008-14. Store at 20° to 25°C (68° to 77°F). [See USP controlled room ...
-
PRINCIPAL DISPLAY PANEL
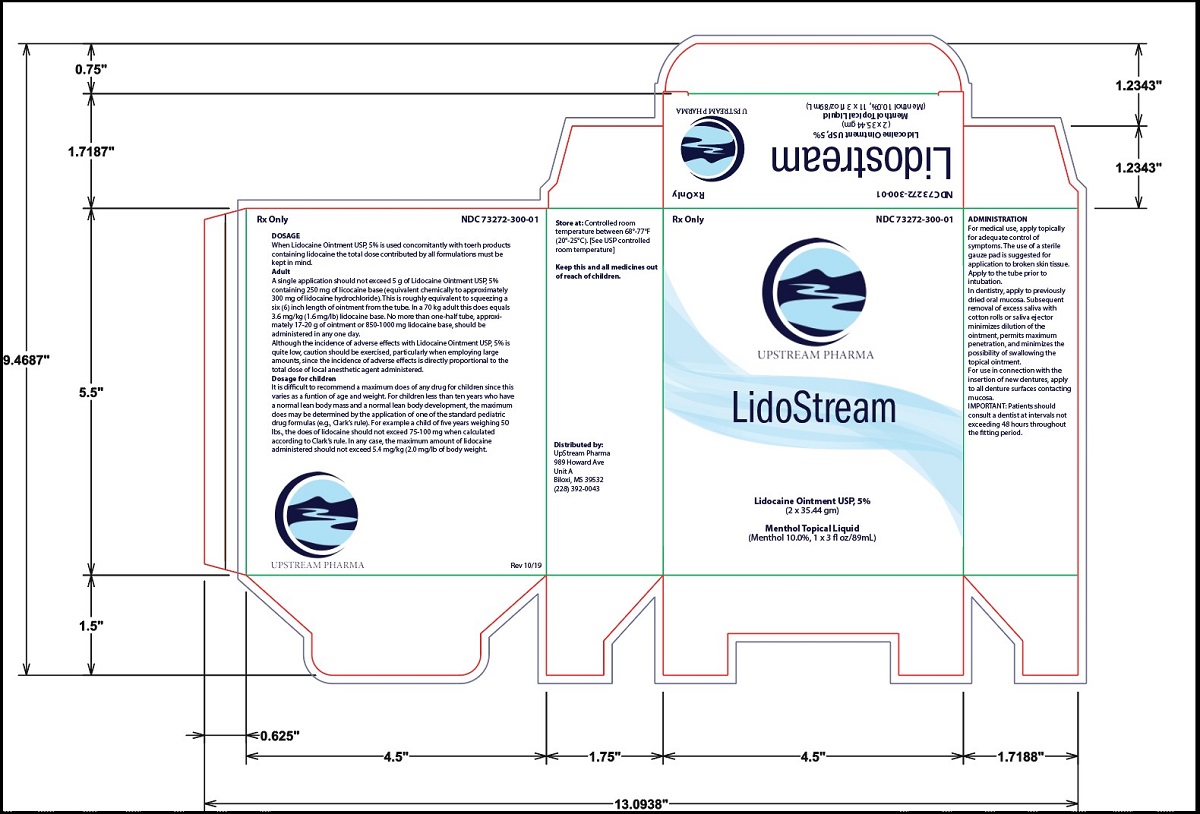
-
INGREDIENTS AND APPEARANCEProduct Information