Label: TAKHZYRO- lanadelumab-flyo solution
TAKHZYRO- lanadelumab-flyo injection, solution
- NDC Code(s): 47783-644-01, 47783-645-01, 47783-646-01
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAKHZYRO® safely and effectively. See full prescribing information for TAKHZYRO®. TAKHZYRO® (lanadelumab-flyo) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETAKHZYRO® is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients aged 2 years and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Adult and Pediatric Patients 12 Years of Age and Older - The recommended starting dosage in adult and pediatric patients 12 years of age and older is 300 mg ...
-
3 DOSAGE FORMS AND STRENGTHSTAKHZYRO is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution available in the following presentations. Injection: 150 mg/1 mL (150 mg/mL) solution ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions have been observed. In case of a severe hypersensitivity reaction, discontinue TAKHZYRO administration and institute appropriate ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo dedicated drug interaction studies have been conducted [see Clinical Pharmacology (12.3)]. 7.1 Drug-Laboratory Test Interactions - Coagulation tests - TAKHZYRO can increase activated ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on TAKHZYRO use in pregnant women to inform any drug associated risks. Monoclonal antibodies such as lanadelumab-flyo are transported ...
-
10 OVERDOSAGEThere is no clinical experience with overdosage of TAKHZYRO.
-
11 DESCRIPTIONLanadelumab-flyo, a plasma kallikrein inhibitor, is a non-plasma derived, recombinant, fully human, monoclonal antibody (IgG1/κ-light chain) produced in Chinese Hamster Ovary (CHO) cells. Based on ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lanadelumab-flyo is a fully human monoclonal antibody (IgG1/κ-light chain) that binds plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to evaluate the carcinogenic potential of lanadelumab-flyo. Published literature supports ...
-
14 CLINICAL STUDIESTrial 1 (NCT02586805) The efficacy of TAKHZYRO for the prevention of angioedema attacks in patients 12 years of age and older with Type I or II HAE was demonstrated in a multicenter ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - TAKHZYRO (lanadelumab-flyo) injection is a ready-to-use, clear to slightly opalescent, colorless to slightly yellow solution supplied in the following ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Hypersensitivity - Advise patients to seek immediate medical attention if they ...
-
SPL UNCLASSIFIED SECTIONFor more information, visit www.TAKHZYRO.com - Manufactured by - Takeda Pharmaceuticals U.S.A., Inc. Cambridge, MA 02142 - U.S. License No. 1898 - TAKHZYRO is a registered trademark of Dyax Corp ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 1/2025 - PATIENT INFORMATION - TAKHZYRO® (tak-ZYE-roe) (lanadelumab-flyo) injection, for ...
-
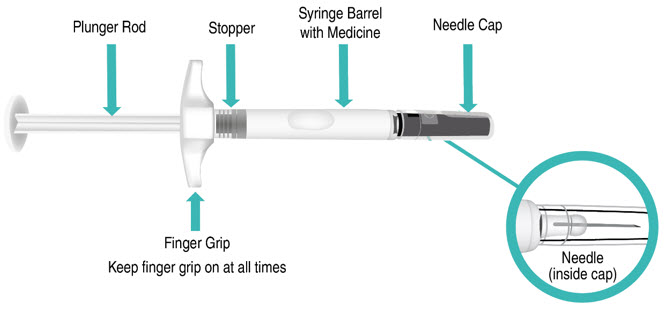
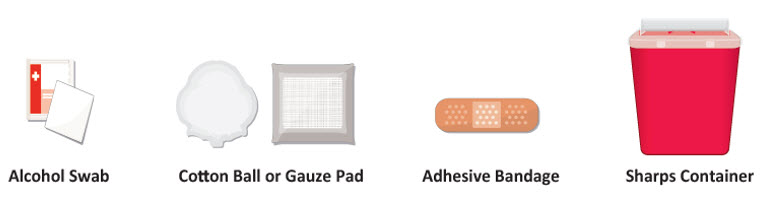
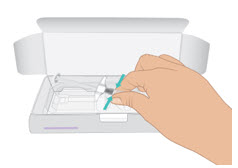
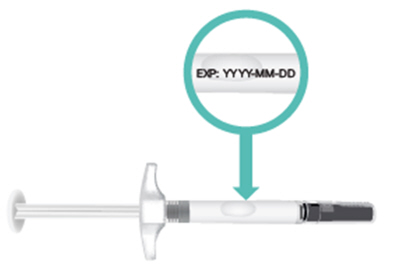
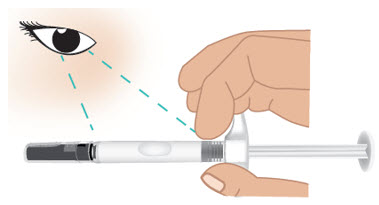
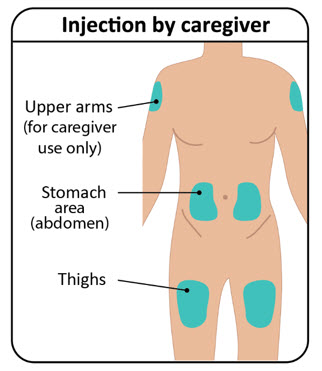
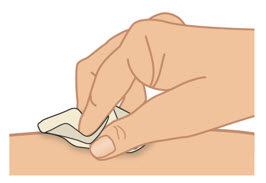
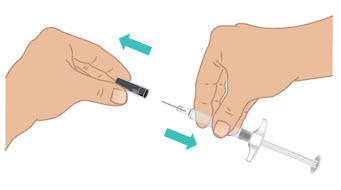
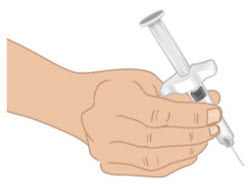
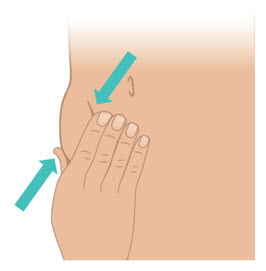
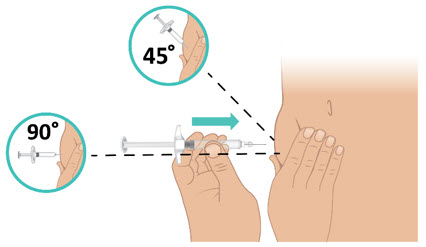
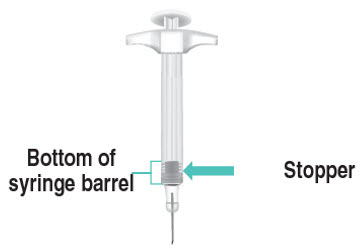
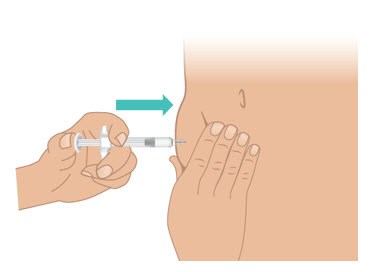
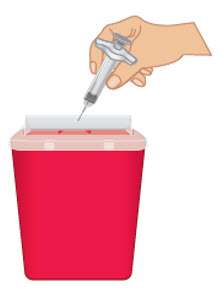
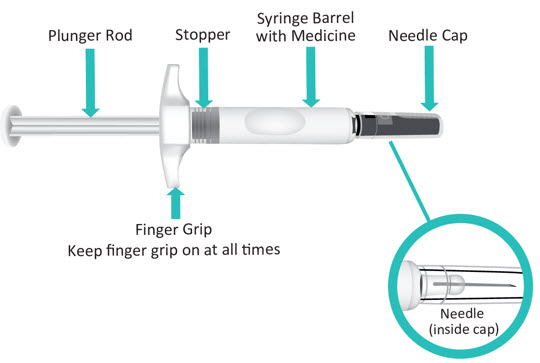
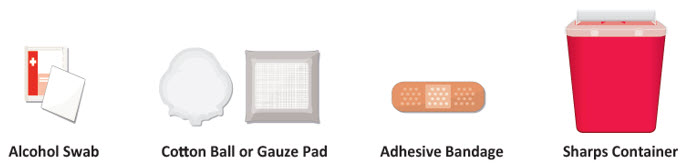
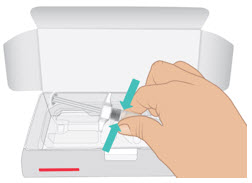
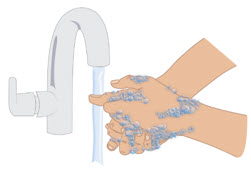
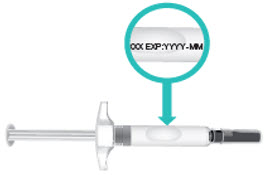
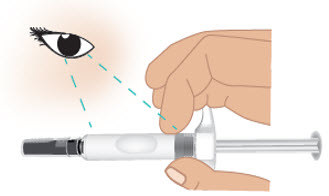
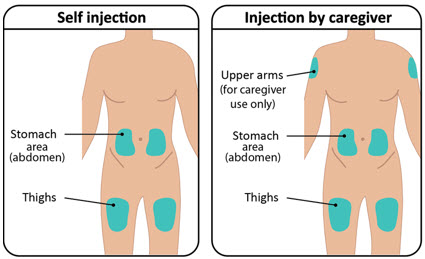
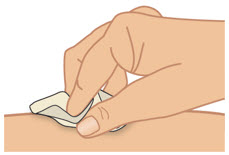
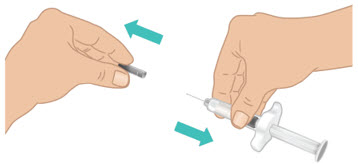
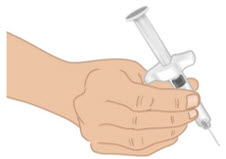
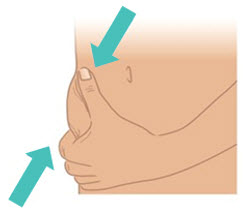
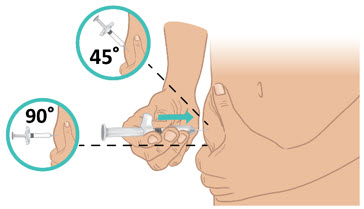
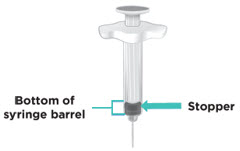
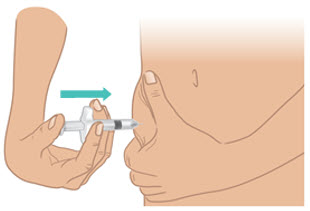
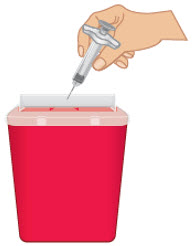
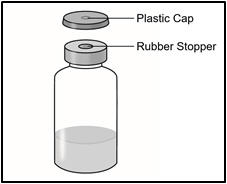
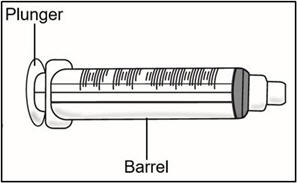
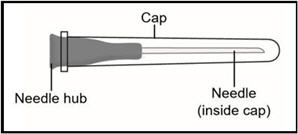
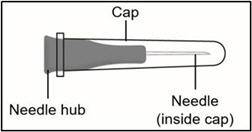
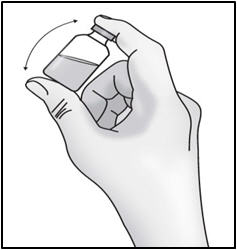
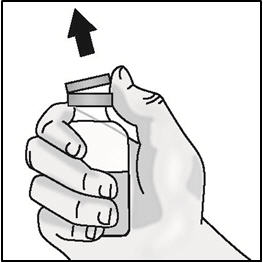
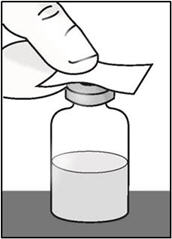
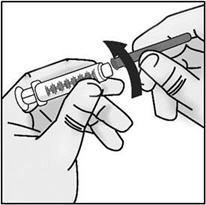
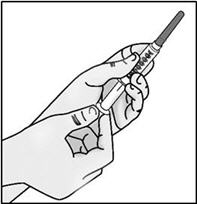
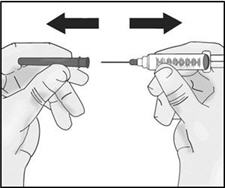
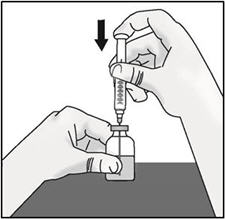
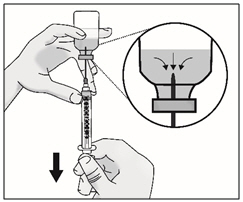
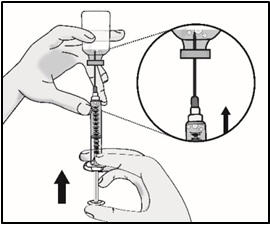
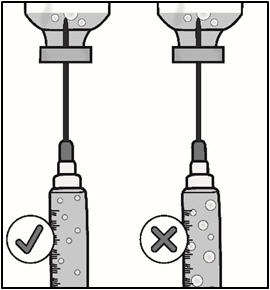
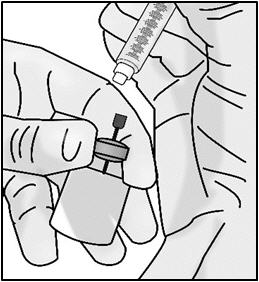
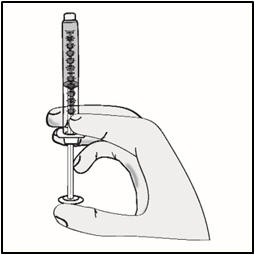
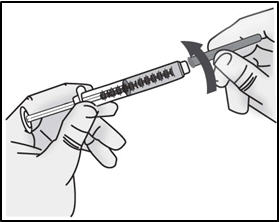
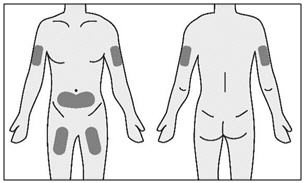
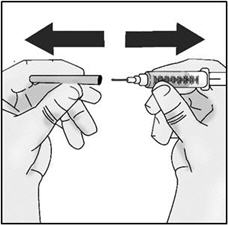
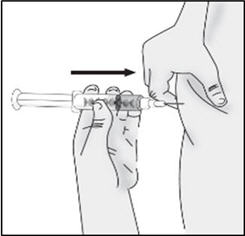
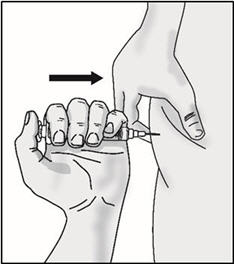
INSTRUCTIONS FOR USETAKHZYRO® (tak-ZYE-roe) (lanadelumab-flyo) injection, for subcutaneous use - single-dose 1 mL prefilled syringe - This Instructions for Use contains information on how to inject TAKHZYRO. Please make ...
-
INSTRUCTIONS FOR USETAKHZYRO® (tak-ZYE-roe) (lanadelumab-flyo) injection, for subcutaneous use - single-dose prefilled syringe - This Instructions for Use contains information on how to inject TAKHZYRO. Please make sure ...
-
INSTRUCTIONS FOR USETAKHZYRO® (tak-ZYE-roe)(lanadelumab-flyo)injection, for subcutaneous useBe sure that you read, understand, and follow the Instructions for Use before injecting TAKHZYRO. A healthcare provider should show you how to prepare and inject TAKHZYRO properly before you use ...
-
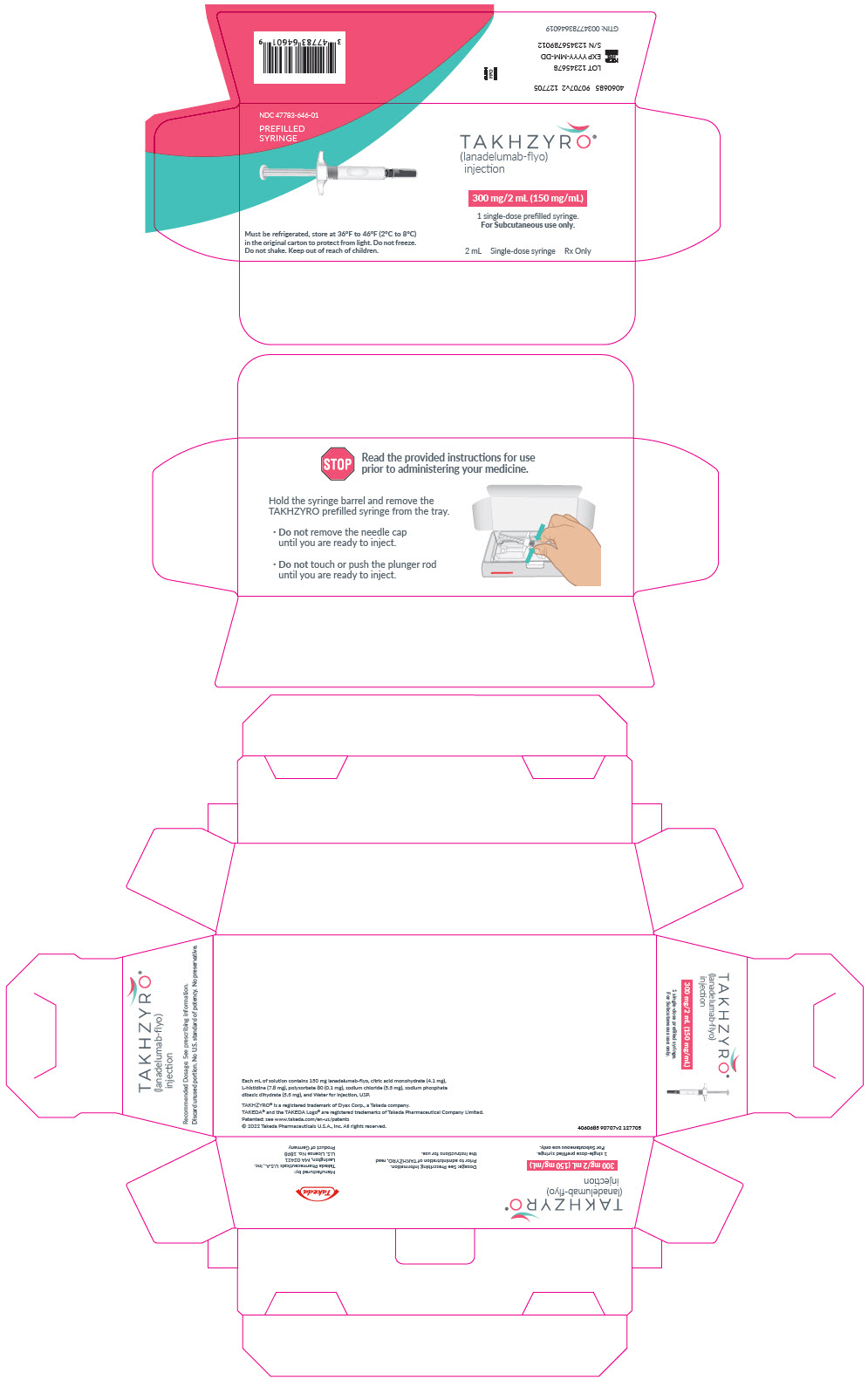
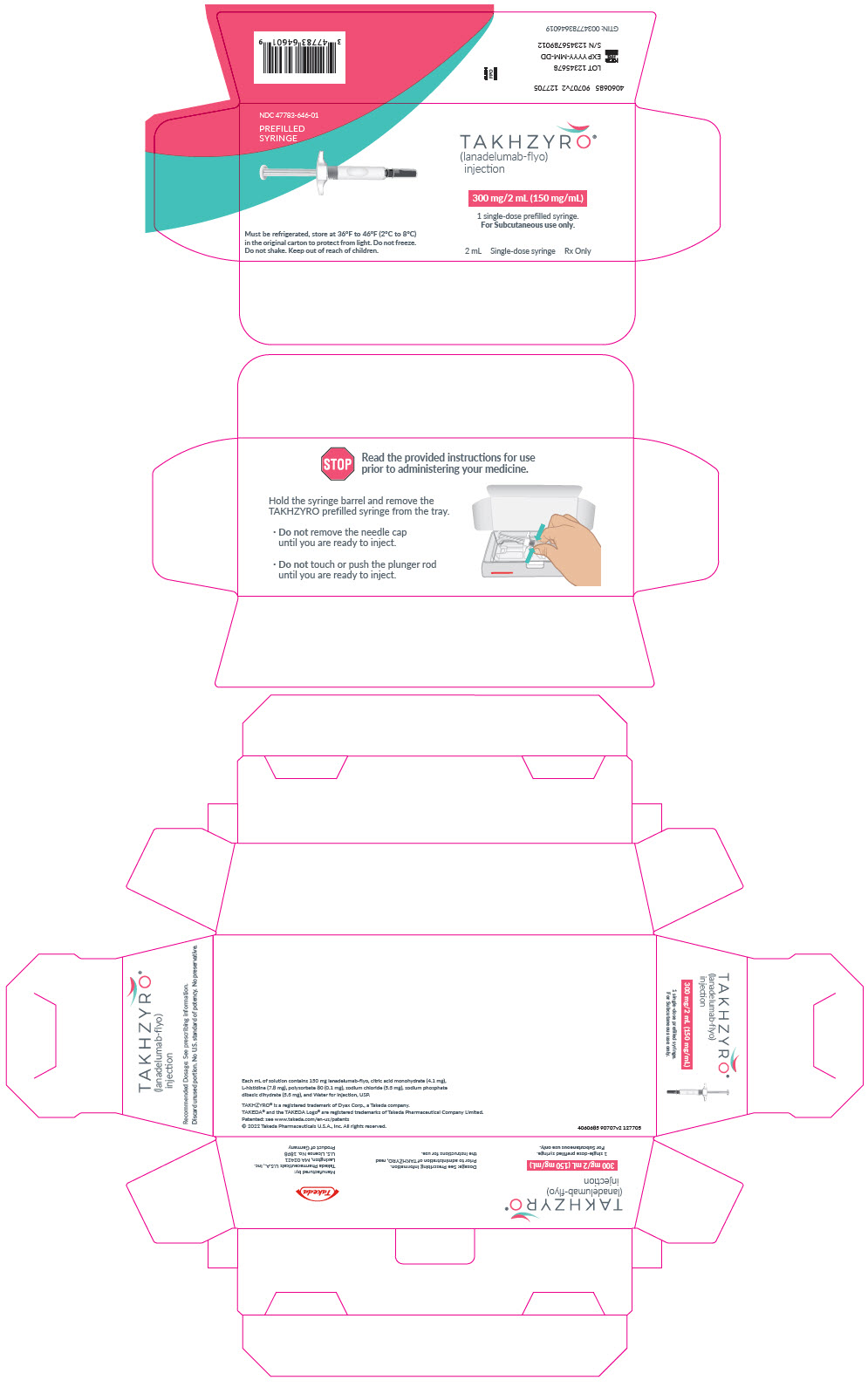
PRINCIPAL DISPLAY PANEL - 2 mL Syringe CartonNDC 47783-646-01 - PREFILLED - SYRINGE - TAKHZYRO® (lanadelumab-flyo) injection - 300 mg/2 mL (150 mg/mL) 1 single-dose prefilled syringe. For Subcutaneous use only. Must be refrigerated, store ...
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial CartonRx Only - NDC 47783-644-01 - TAKHZYRO® (lanadelumab-flyo) injection - 300 mg/2 mL (150 mg/mL) Single-dose Vial, for Subcutaneous use only. 2 mL - 1 single-dose vial. Takeda
-
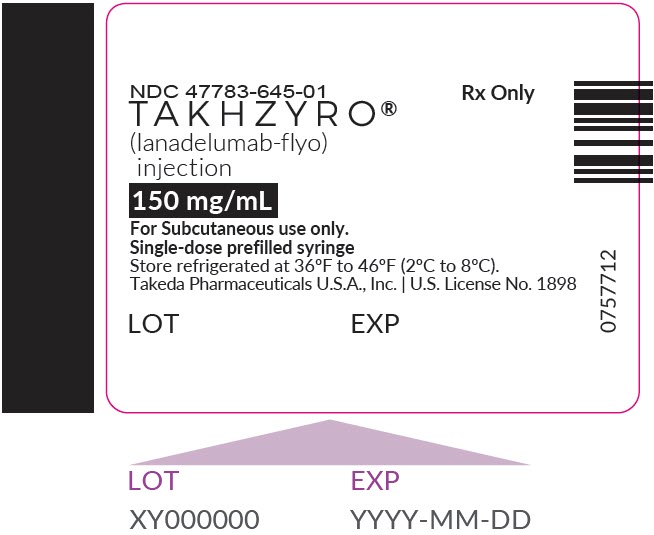
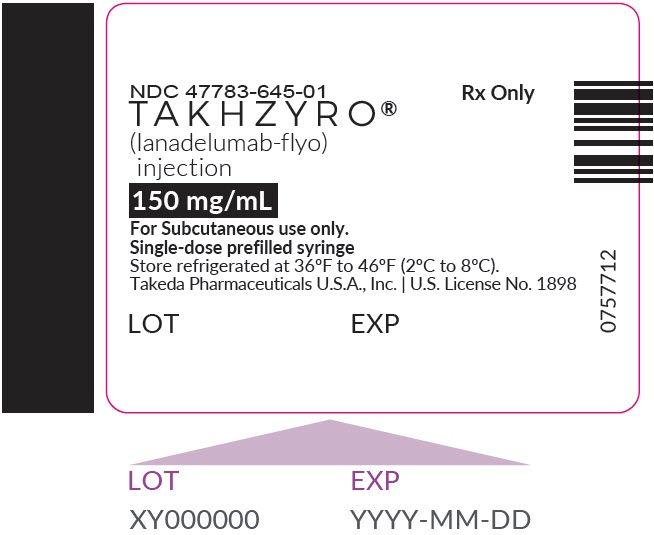
PRINCIPAL DISPLAY PANEL - 150 mg/mL Syringe LabelNDC 47783-645-01 - TAKHZYRO® (lanadelumab-flyo) injection - 150 mg/mL - For Subcutaneous use only. Single-dose prefilled syringe - Store refrigerated at 36°F to 46°F (2°C to 8°C). Takeda Pharmaceuticals ...
-
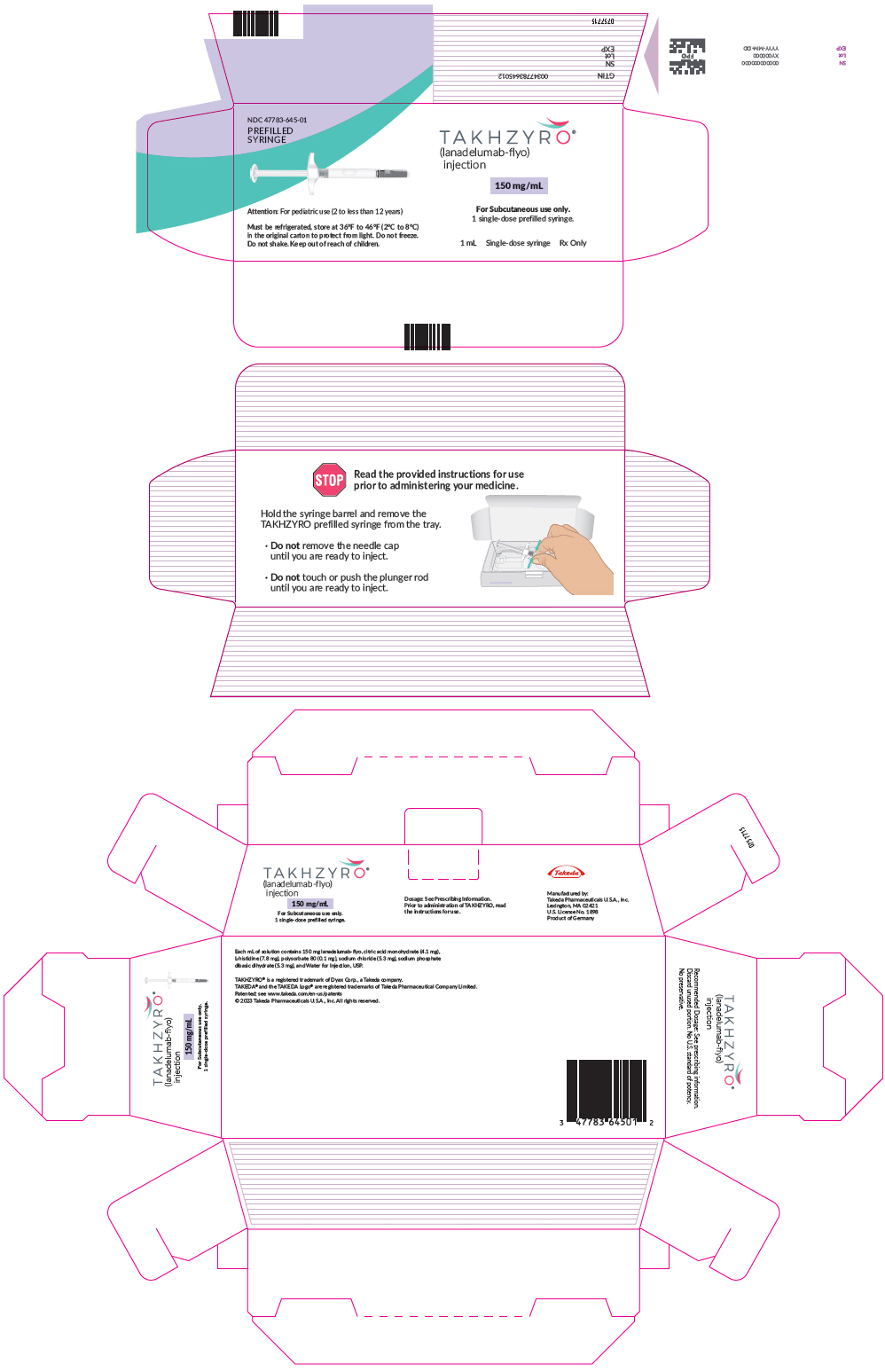
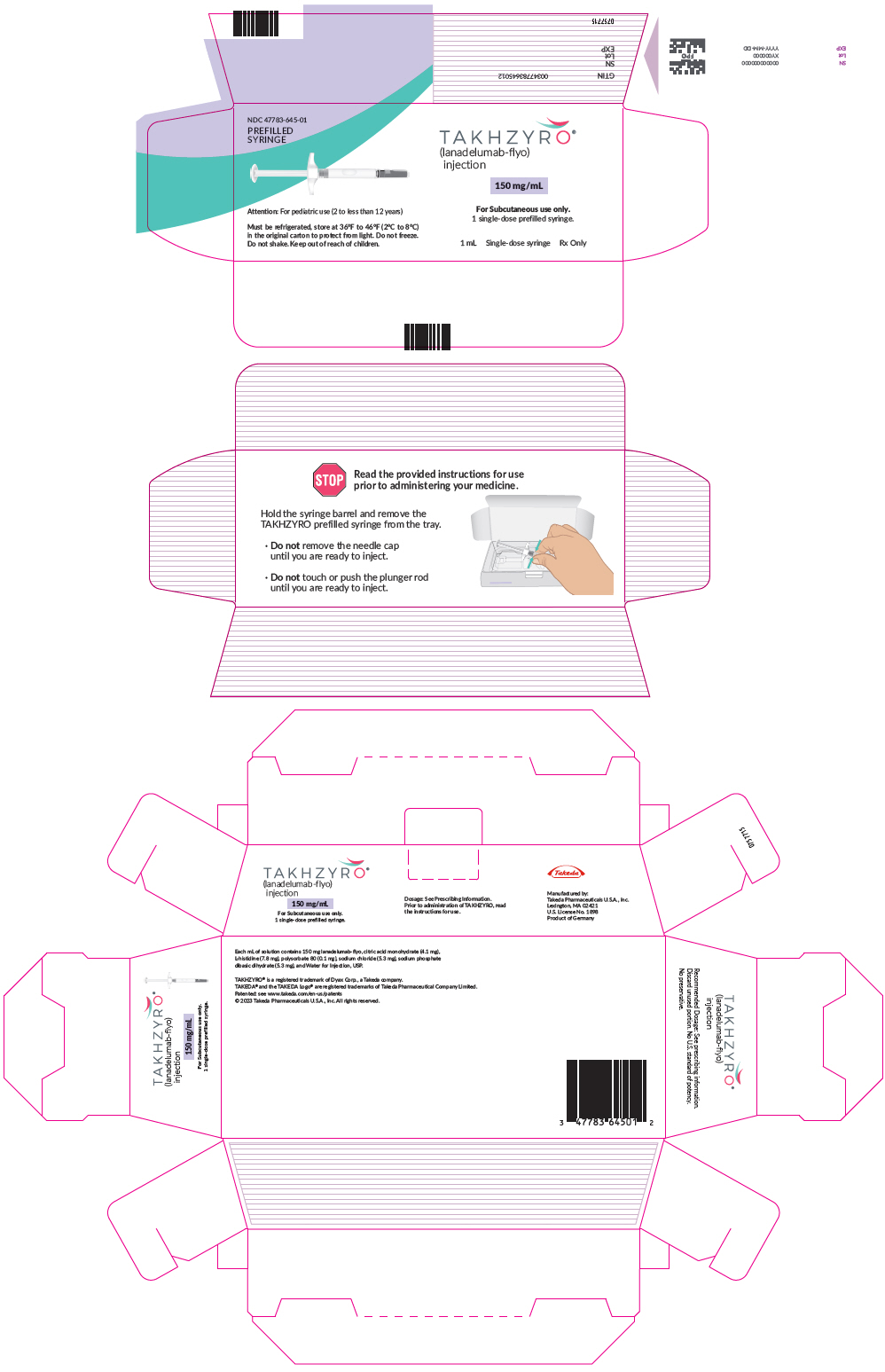
PRINCIPAL DISPLAY PANEL - 150 mg/mL Syringe CartonNDC 47783-645-01 - PREFILLED - SYRINGE - TAKHZYRO® (lanadelumab-flyo) injection - 150 mg/mL - Attention: For pediatric use (2 to less than 12 years) For Subcutaneous use only. 1 single-dose ...
-
INGREDIENTS AND APPEARANCEProduct Information


 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.