Label: KRISTALOSE- lactulose powder, for solution
- NDC Code(s): 66220-719-01, 66220-719-30, 66220-729-01, 66220-729-30
- Packager: Cumberland Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 17, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
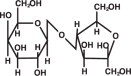
KRISTALOSE (lactulose) is a synthetic disaccharide in the form of crystals for reconstitution prior to use for oral administration Each 10 g of lactulose contains less than 0.3 g galactose and ...
-
CLINICAL PHARMACOLOGY
KRISTALOSE® (LACTULOSE) is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral ...
-
INDICATIONS AND USAGE
KRISTALOSE® (LACTULOSE) For Oral Solution is indicated for the treatment of constipation. In patients with a history of chronic constipation, lactulose therapy increases the number of bowel ...
-
CONTRAINDICATIONS
Since KRISTALOSE® (LACTULOSE) For Oral Solution contains galactose (less than 0.3 g/10 g as a total sum with lactose), it is contraindicated in patients who require a low galactose diet.
-
WARNINGS
A theoretical hazard may exist for patients being treated with lactulose who may be required to undergo electrocautery procedures during proctoscopy or colonoscopy. Accumulation of H2 gas in ...
-
PRECAUTIONS
General - Since KRISTALOSE® (LACTULOSE) For Oral Solution contains galactose and lactose (less than 0.3 g/10 g as a total sum), it should be used with caution in diabetics. Information for ...
-
ADVERSE REACTIONS
Precise frequency data are not available. Initial dosing may produce flatulence and intestinal cramps, which are usually transient. Excessive dosage can lead to diarrhea with potential ...
-
OVERDOSAGE
Signs and Symptoms - There have been no reports of accidental overdosage. In the event of overdosage, it is expected that diarrhea and abdominal cramps would be the major symptoms. Medication ...
-
DOSAGE AND ADMINISTRATION
The usual adult dosage is 10 g to 20 g of lactulose daily. The dose may be increased to 40 g daily if necessary. Twenty-four to 48 hours may be required to produce a normal bowel movement.
-
DIRECTIONS FOR PREPARATION
Dissolve contents of packet in half a glass (4 ounces) of water. When Lactulose For Oral Solution is dissolved in water, the resulting solution may be colorless to a slightly pale yellow ...
-
HOW SUPPLIED
KRISTALOSE® (LACTULOSE) For Oral Solution is available in single dose packets of 10 g (NDC 66220-719-01) and single dose packets of 20 g (NDC 66220-729-01). The packets are supplied as ...
-
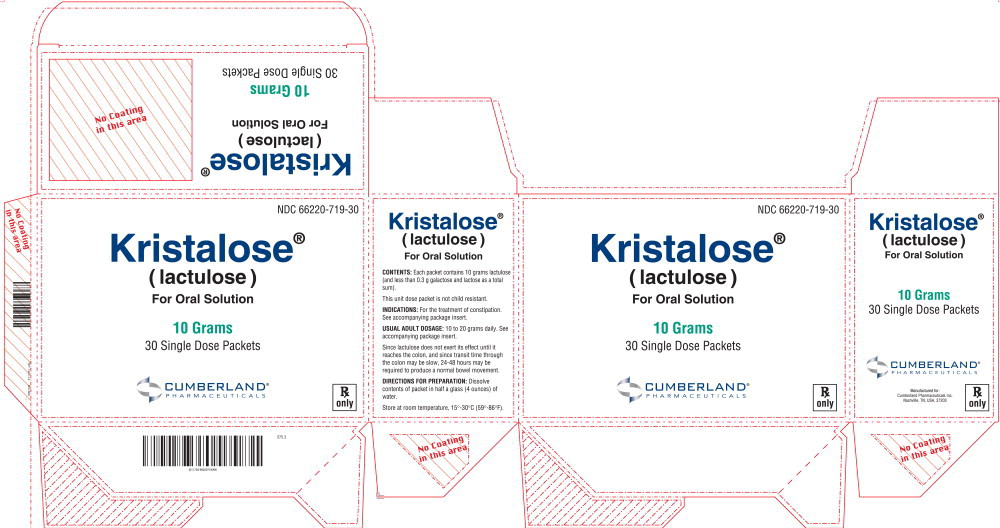
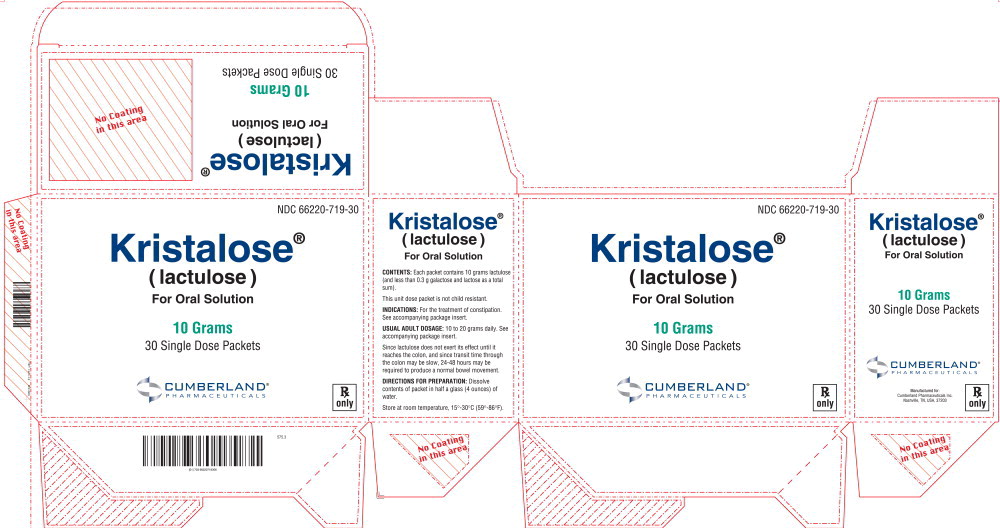
PRINCIPAL DISPLAY PANELPrincipal Display panel – 10 Grams Carton Label - NDC 66220-719-30 - Kristalose® ( lactulose ) For Oral Solution - 10 Grams - 30 Single Dose Packets - CUMBERLAND® PHARMACEUTICALS - Rx only
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 10 Grams Pouch Label - NDC 66220-719-01 - Kristalose® ( lactulose ) For Oral Solution - CUMBERLAND® PHARMACEUTICALS - 10 Grams - Single Dose Packet - Rx only - CONTENTS: Each ...
-
PRINCIPAL DISPLAY PANELPrincipal Display panel – 20 Grams Carton Label - NDC 66220-729-30 - Kristalose® ( lactulose ) For Oral Solution - 20 Grams - 30 Single Dose Packets - CUMBERLAND® PHARMACEUTICALS - Rx only
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 20 Grams Pouch Label - NDC 66220-729-01 - Kristalose® ( lactulose ) For Oral Solution - CUMBERLAND® PHARMACEUTICALS - 20 Grams - Single Dose Packet - Rx only - CONTENTS: Each ...
-
INGREDIENTS AND APPEARANCEProduct Information