Label: KOSELUGO- selumetinib capsule
- NDC Code(s): 0310-0610-28, 0310-0610-60, 0310-0625-28, 0310-0625-60
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KOSELUGO safely and effectively. See full prescribing information for KOSELUGO. KOSELUGO® (selumetinib) capsules, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE KOSELUGO is indicated for the treatment of pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN).
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - The recommended dosage of KOSELUGO is 25 mg/m2 orally twice daily (approximately every 12 hours) until disease progression or unacceptable toxicity. KOSELUGO can be ...
-
3 DOSAGE FORMS AND STRENGTHS Capsules: • 10 mg: white to off-white, opaque, hard capsule sealed with a clear band and marked with “SEL 10” in black ink. • 25 mg: blue, opaque, hard capsule sealed with a clear band and marked ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Cardiomyopathy - Cardiomyopathy, defined as a decrease in left ventricular ejection fraction (LVEF) ≥ 10% below baseline, occurred in 23% of 74 pediatric patients who received KOSELUGO in ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Cardiomyopathy [see Warnings and Precautions (5.1)] • Ocular toxicity [see Warnings and ...
-
7 DRUG INTERACTIONS 7.1 Effect of Other Drugs on KOSELUGO - Strong or Moderate CYP3A4 Inhibitors or Fluconazole - Clinical Impact - • Concomitant use of KOSELUGO with a strong or moderate CYP3A4 inhibitor ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], KOSELUGO can cause fetal harm when administered to a pregnant ...
-
10 OVERDOSAGE Dialysis is not helpful as KOSELUGO is highly protein bound and is extensively metabolized.
-
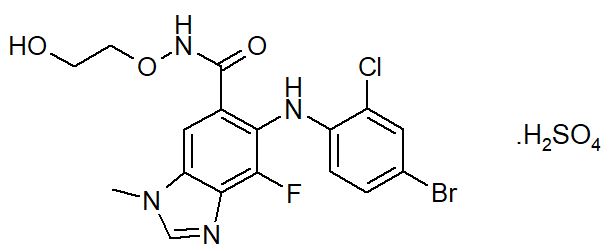
11 DESCRIPTION Selumetinib is a kinase inhibitor. The chemical name is 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-6-[(2-hydroxyethoxy)carbamoyl]-1-methyl-1H-benzimidazol-3-ium hydrogen sulfate. The molecular ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Selumetinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Selumetinib was not carcinogenic in a 6-month study in rasH2 transgenic mice at exposures 24-times (males) and ...
-
14 CLINICAL STUDIES 14.1 Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN) The efficacy of KOSELUGO was evaluated in SPRINT Phase II Stratum 1, an open-label, multicenter, single arm ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - Strength - Description - Capsules per Bottle - NDC Number - 10 mg - White to off-white, opaque, hard capsule sealed with a clear band and marked with “SEL 10” in black ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Cardiomyopathy - Advise patients and caregivers that KOSELUGO can cause a reduction in LVEF and to immediately ...
-
PATIENT PACKAGE INSERTPatient Information - KOSELUGO™ (ko-SEL-u-go) (selumetinib) capsules - What is KOSELUGO? KOSELUGO is a prescription medicine that is used to treat children 2 years of age and older with ...
-
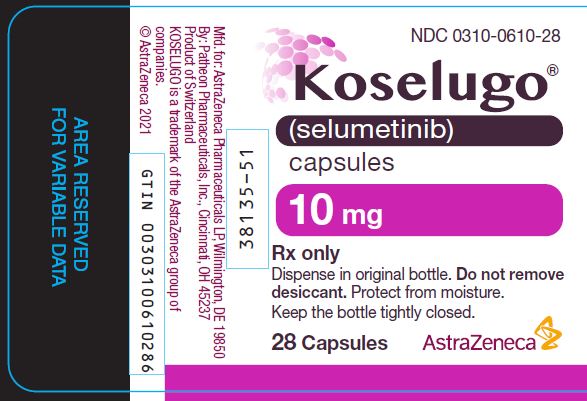
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 10mg NDC 0310-0610-28 - Koselugo® (selumetinib) capsules - 10 mg - Rx only - Dispense in original bottle. Do not remove - desiccant. Protect from moisture. Keep the bottle tightly closed. 28 ...
-
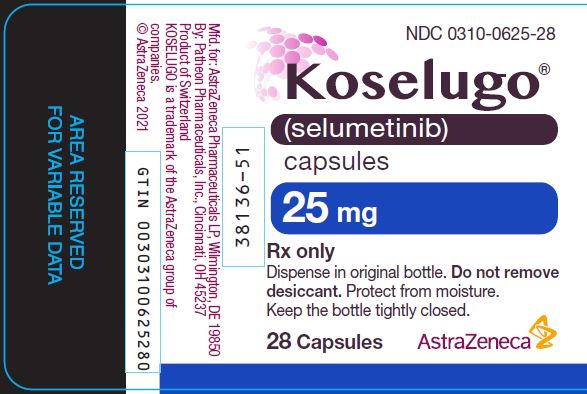
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 25mg NDC 0310-0625-28 - Koselugo® (selumetinib) capsules - 25 mg - Rx only - Dispense in original bottle. Do not remove - desiccant. Protect from moisture. Keep the bottle tightly closed. 28 ...
-
INGREDIENTS AND APPEARANCEProduct Information