Label: KITABIS PAK- tobramycin solution
- NDC Code(s): 24492-850-56
- Packager: Pari Respiratory Equipment, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KITABIS® PAK safely and effectively. See full prescribing information for KITABIS PAK. KITABIS PAK (tobramycin inhalation solution) ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
KITABIS PAK (co-packaging of tobramycin inhalation solution and PARI LC PLUS Reusable Nebulizer) is indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age ...
-
2 DOSAGE AND ADMINISTRATION
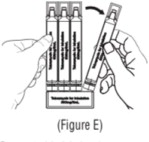
2.1 Dosing Information - KITABIS PAK is a co-packaging of tobramycin inhalation solution ampules with a PARI LC PLUS Reusable Nebulizer. Administer as follows: One single-use ampule (300 mg/5 ...
-
3 DOSAGE FORMS AND STRENGTH
Inhalation solution: 300 mg/5 mL in a single-use ampule
-
4 CONTRAINDICATIONS
Tobramycin inhalation solution is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
-
5 WARNINGS AND PRECAUTIONS
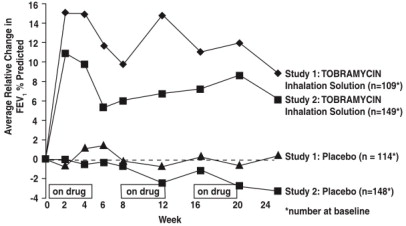
5.1 Bronchospasm - Bronchospasm can occur with inhalation of tobramycin inhalation solution. In clinical studies with tobramycin inhalation solution, changes in FEV1 measured after the inhaled ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling: Bronchospasm [see Warnings and Precautions (5.1)] Ototoxicity [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS
7.1 Drugs with Neurotoxic, Nephrotoxic or Ototoxic Potential - Concurrent and/or sequential use of tobramycin inhalation solution with other drugs with neurotoxic, nephrotoxic, or ototoxic ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Aminoglycosides can cause fetal harm. Published literature reports that use of streptomycin, an aminoglycoside, can cause total, irreversible, bilateral ...
-
10 OVERDOSAGE
Signs and symptoms of acute toxicity from overdosage of IV tobramycin might include dizziness, tinnitus, vertigo, loss of high-tone hearing acuity, respiratory failure, and neuromuscular blockade ...
-
11 DESCRIPTION
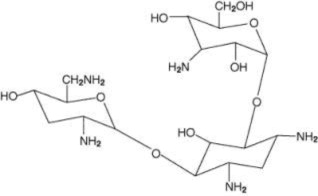
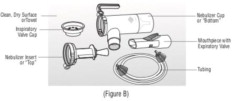
KITABIS PAK contains tobramycin inhalation solution, USP and the PARI LC PLUS Reusable Nebulizer (PARI LC PLUS). Tobramycin inhalation solution is a sterile, clear, slightly yellow, non-pyrogenic ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Tobramycin is an aminoglycoside antibacterial [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - Tobramycin inhalation solution contains ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A two-year rat inhalation toxicology study to assess carcinogenic potential of tobramycin inhalation solution has been completed. Rats ...
-
14 CLINICAL STUDIES
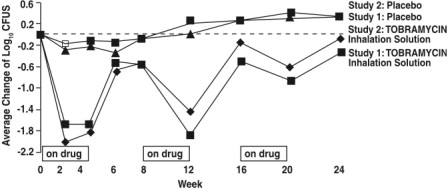
Two identically designed, double-blind, randomized, placebo-controlled, parallel group, 24-week clinical studies (Study 1 and Study 2) at a total of 69 cystic fibrosis centers in the United States ...
-
15 REFERENCES
Neu HC. Tobramycin: an overview. [Review]. J Infect Dis 1976; Suppl 134:S3-19. Weber A, Smith A, Williams-Warren J et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr ...
-
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied - KITABIS PAK co-packaged kit (NDC 24492-850-56) is available in cartons containing one reusable PARI LC PLUS nebulizer (Model No.: 022B81-T) and 14 tobramycin inhalation ...
-
17 PATIENT COUNSELING INFORMATION
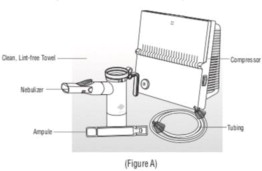
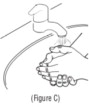
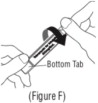
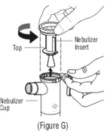
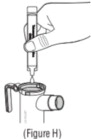
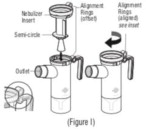
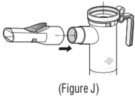
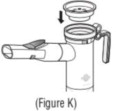
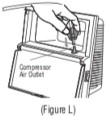
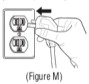
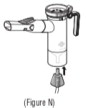
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Instructions for Administration - Instruct patients to read the Instructions for ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - KITABIS® PAK (Ki TAH biss Pak) (tobramycin inhalation solution) for oral inhalation - Read this Patient Information before you start using KITABIS PAK and each time ...
-
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - KITABIS PAK (Ki TAH biss Pak) (tobramycin inhalation solution), for oral inhalation - Follow the instructions below for using KITABIS PAK which contains tobramycin ...
-
Principal Display Panel - Kitabis Pak
NDC: 24492-850-56 - PARI - KitabisTM Pak - Tobramycin Inhalation Solution, USP - Store in Refrigerator - Includes One PARI LC PLUS Nebulizer Set - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information