Label: KIT FOR THE PREPARTION OF TECHNETIUM TC99M SULFUR COLLOID- technetium tc 99m sulfur colloid kit
- NDC Code(s): 45567-0030-1
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection. See full prescribing information for Kit for the ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETechnetium Tc 99m Sulfur Colloid Injection is indicated: In adults, to assist in the: localization of lymph nodes draining a primary tumor in patients with breast cancer or malignant melanoma ...
-
2 DOSAGE AND ADMINISTRATIONTechnetium Tc 99m Sulfur Colloid Injection emits radiation. Use procedures to minimize radiation exposure. Measure patient dose by a suitable radioactivity calibration system immediately before ...
-
3 DOSAGE FORMS AND STRENGTHSKit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied in a package that contains 5 kits. All components of a kit are sterile and non-pyrogenic. Each 10mL multi-dose ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylactic Reactions - Anaphylactic reactions with bronchospasm, hypotension, urticaria and rare fatalities have occurred following intravenously administered Technetium Tc 99m Sulfur ...
-
6 ADVERSE REACTIONSThe most frequently reported adverse reactions, across all categories of use and routes of administration, include rash, allergic reaction, urticaria, anaphylaxis/anaphylactic shock, and ...
-
7 DRUG INTERACTIONSSpecific drug-drug interactions have not been studied.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with Technetium Tc 99m Sulfur Colloid Injection use in pregnant women have not identified a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEThe clinical consequences of overdosing with Technetium Tc 99m Sulfur Colloid Injection are not known.
-
11 DESCRIPTIONKit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection contains a multi-dose Reaction Vial, a Solution A vial and a Solution B vial which contain the sterile non-pyrogenic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Technetium Tc 99m decays by isomeric transition, emitting a photon that can be detected for imaging purposes. [see Description (11.1)] Following subcutaneous ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies to evaluate the carcinogenicity, mutagenesis, or reproductive toxicity potentials of Technetium Tc 99m Sulfur Colloid ...
-
14 CLINICAL STUDIES14.1 Tracer Localization to Lymph Nodes in Breast Cancer - A systematic review of 43 publications examined procedures that used the injection of Technetium Tc 99m Sulfur Colloid Injection and a ...
-
15 REFERENCESBergqvist L, Strand S-E, Persson B, et al. Dosimetry in Lymphoscintigraphy of Tc 99m Antimony Sulfide Colloid, J Nucl Med., 23: 698-705, 1982. Summary of Current Radiation Dose Estimates to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied in a package that contains 5 kits. All kit components are sterile and non-pyrogenic. Each 10mL multi-dose Reaction ...
-
17 PATIENT COUNSELING INFORMATIONInform patients they may experience a burning sensation at the injection site. Inform lactating woman to pump and discard breast milk for 24 hours after administration of Technetium Tc 99m Sulfur ...
-
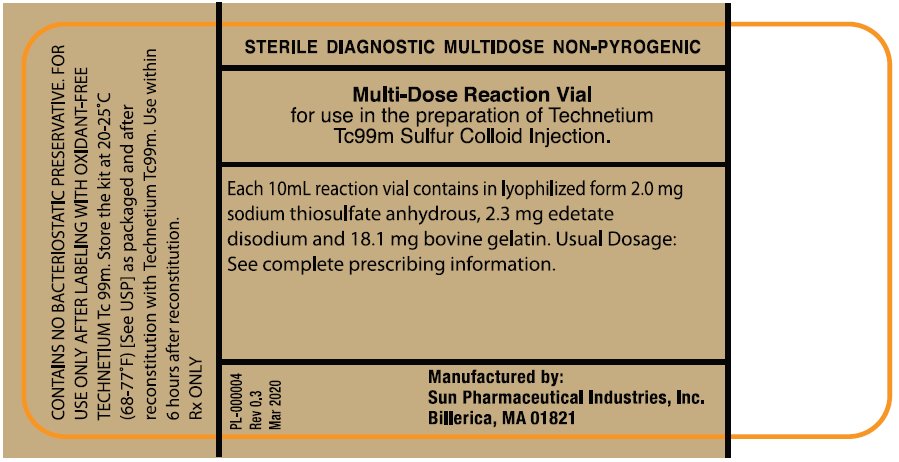
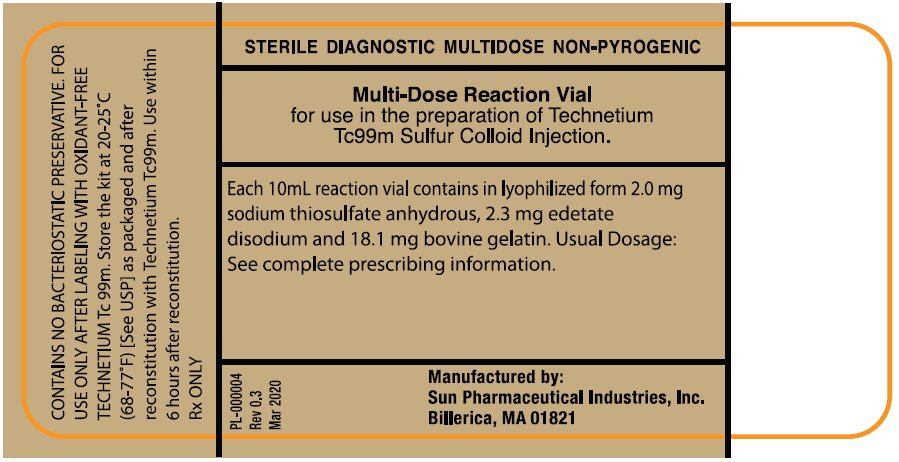
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - VIAL CONTAINER (PART 1 - 10mL multi-dose Reaction Vial)NDC 045567-0030-1 - STERILE DIAGNOSTIC MULTIDOSE NON-PYROGENIC - Multi-Dose Reaction Vial - for use in the Preparation of Technetium Tc 99m Sulfur Colloid Injection. Each 10 mL reaction vial ...
-
PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - VIAL CONTAINER (PART 2 - 3mL Solution A Vial)NDC 045567-0030-1 - A - Solution A vial contains 1.8mL sterile, non pyrogenic 0.148 N hydrochloric acid solution. To be used only with the Sulfur Colloid Multi-dose Reaction Vial. Single Use ...
-
PACKAGE/LABEL - PRINCIPAL DISPALY PANEL - VIAL CONTAINER (PART 3 - 3mL Solution B Vial)NDC 045567-0030-1 - B - Solution B vial contains 1.8mL sterile, non pyrogenic aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. To be used only with the ...
-
PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - RADIATION LABELCAUTION RADIOACTIVE MATERIAL - STERILE, NON-PYROGENIC, DIAGNOSTIC MULTIDOSE TECHNETIUM Tc 99m SULFUR COLLOID - Subcutaneous, Intravenous, Oral, and Intraperitoneal Use - Total MBq ...
-
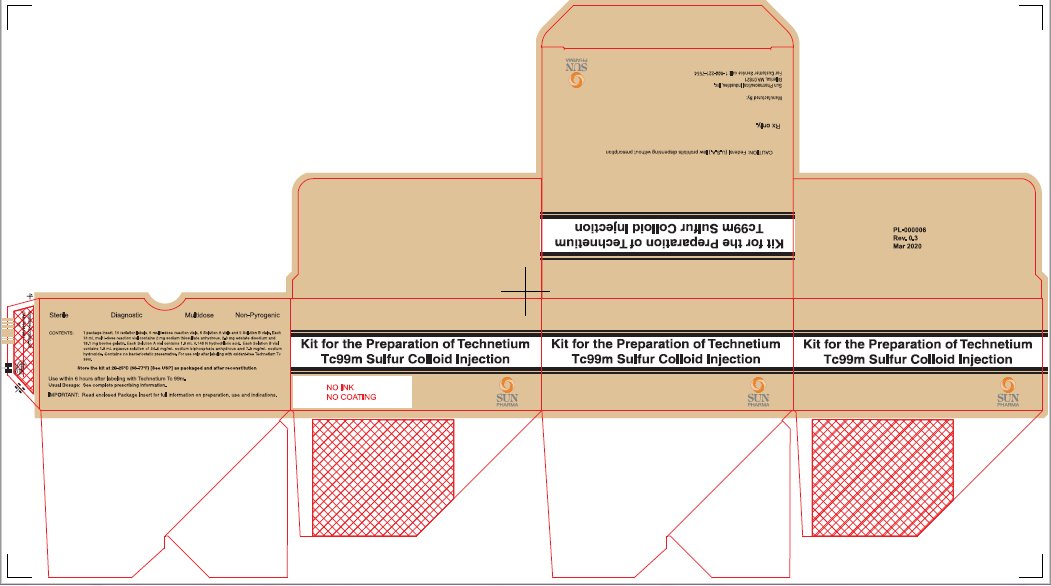
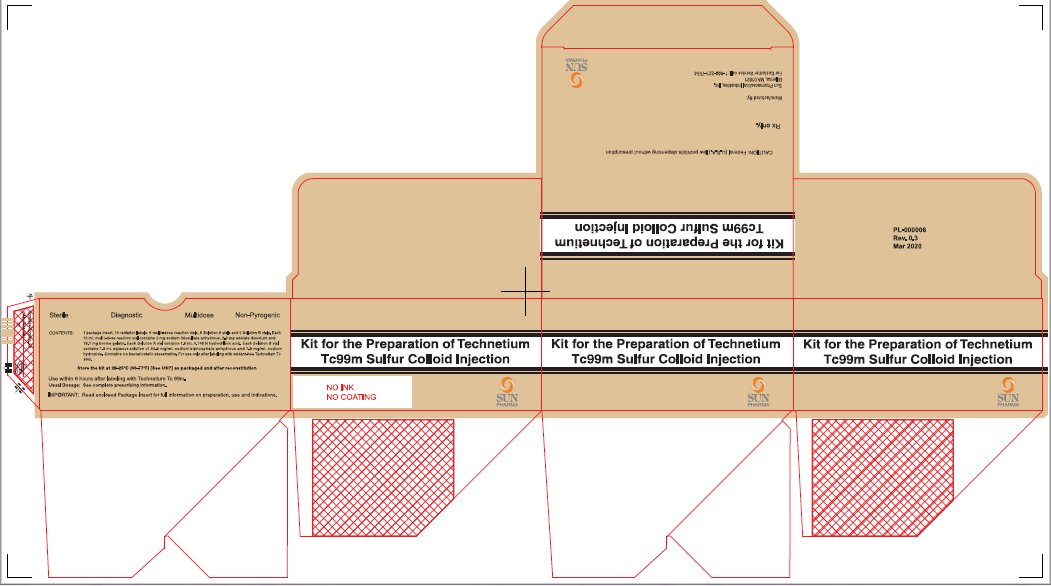
PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - 5 VIAL BOXNDC 045567-0030-1 - Kit for the Preparation of Technetium Tc99m Sulfur Colloid Injection - CAUTION: Federal (U.S.A.) law prohibits dispensing without prescription - Rx only. Manufactured By: Sun ...
-
INGREDIENTS AND APPEARANCEProduct Information