Label: KIONEX- sodium polystyrene sulfonate suspension
- NDC Code(s): 62559-356-01, 62559-356-60
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Cation-Exchange ResinRx only
-
DESCRIPTION
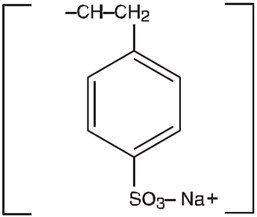
Kionex® (Sodium Polystyrene Sulfonate Suspension USP) can be administered orally or in an enema. It is a raspberry flavored suspension containing 15 grams of cation-exchange resin (sodium ...
-
CLINICAL PHARMACOLOGY As the resin passes along the intestine or is retained in the colon after administration by enema, the sodium ions are partially released and are replaced by potassium ions. For the most part ...
-
INDICATIONS AND USAGE Kionex Suspension is indicated for the treatment of hyperkalemia.
-
CONTRAINDICATIONS Kionex Suspension is contraindicated in the following conditions: patients with hypokalemia, patients with a history of hypersensitivity to polystyrene sulfonate resins, obstructive bowel disease ...
-
WARNINGS Intestinal Necrosis - Cases of intestinal necrosis, which may be fatal, and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in ...
-
PRECAUTIONS Caution is advised when sodium polystyrene sulfonate is administered to patients who cannot tolerate even a small increase in sodium loads (i.e., severe congestive heart failure, severe ...
-
ADVERSE REACTIONS Kionex Suspension may cause some degree of gastric irritation. Anorexia, nausea, vomiting, and constipation may occur especially if high doses are given. Also, hypokalemia, hypocalcemia ...
-
OVERDOSAGE Overdosage may result in electrolyte disturbances including hypokalemia, hypocalcemia, and hypomagnesemia. Biochemical disturbances resulting from overdosage may give rise to clinical signs and ...
-
DOSAGE AND ADMINISTRATION Administer Kionex Suspension at least 3 hours before or 3 hours after other oral medications. Patients with gastroparesis may require a 6 hour separation (see WARNINGS and PRECAUTIONS, Drug ...
-
HOW SUPPLIED Kionex® Suspension is a light brown, raspberry-flavored suspension supplied as follows: NDC 62559-356-01: Case containing 10 unit dose bottles of 60 mL (NDC 62559-356-60) Dispense in a tight ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 62559-356-60 - KIONEX® (Sodium Polystyrene Sulfonate Suspension USP), 15g/60mL - For Oral or Rectal Use - Shake Well Until Fully Suspended - Rx only - 60 mL
-
INGREDIENTS AND APPEARANCEProduct Information