Label: KETODAN- ketoconazole aerosol, foam
- NDC Code(s): 43538-530-10
- Packager: Medimetriks Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use - KETODAN® FOAM safely and effectively. See full prescribing information for KETODAN® FOAM. KETODAN® (KETOCONAZOLE) Foam, 2% For ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEKetodan® Foam, 2% is indicated for the topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older. Limitations of Use - Safety and efficacy of Ketodan ...
-
2. DOSAGE AND ADMINISTRATIONKetodan® Foam, 2% should be applied to the affected area(s) twice daily for four weeks. Hold the container upright, and dispense Ketodan® Foam, 2% into the cap of the can or other cool surface in ...
-
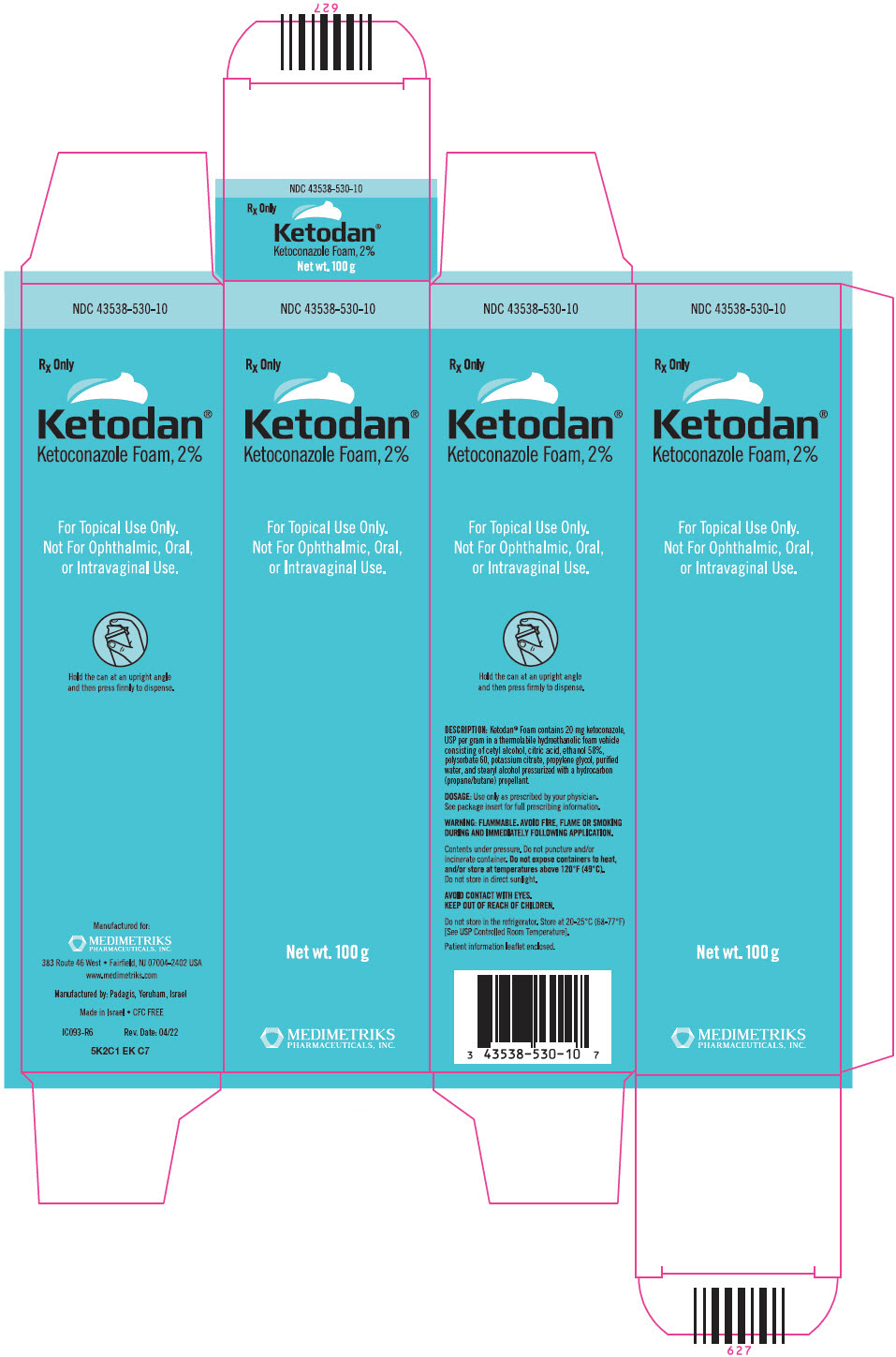
3. DOSAGE FORMS AND STRENGTHSKetodan® Foam, 2% contains 20 mg of ketoconazole, USP per gram, supplied in 100 g containers.
-
4. CONTRAINDICATIONSNone.
-
5. WARNINGS AND PRECAUTIONS5.1 Contact Sensitization - Ketodan® Foam, 2% may result in contact sensitization, including photoallergenicity [see Adverse Reactions (6.2)]. 5.2 Flammable Contents - The contents of Ketodan ...
-
6. ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on ketoconazole foam, 2% use in pregnant women to identify a drug-associated risk of major birth defects, miscarriage or adverse ...
-
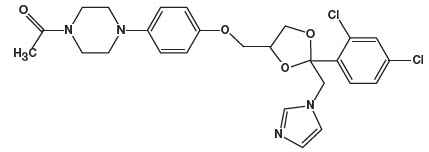
11. DESCRIPTIONKetodan® Foam, 2% contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application. The chemical name for ketoconazole is piperazine ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of ketoconazole in the treatment of seborrheic dermatitis is not known. 12.2 Pharmacodynamics - The pharmacodynamics of Ketodan® Foam, 2% has ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic or photo-carcinogenic potential of ketoconazole foam ...
-
14. CLINICAL STUDIESThe safety and efficacy of ketoconazole foam, 2% were evaluated in a randomized, double-blind, vehicle-controlled trial in subjects 12 years and older with mild to severe seborrheic dermatitis. In ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGKetodan® Foam, 2% contains 20 mg of ketoconazole, USP per gram. The thermolabile hydroethanolic foam is available as follows: NDC 43538-530-10 - 100 g aluminum can - Store at 20° to 25°C (68 ...
-
17. PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Package Insert). Instruct patients on a proper use of Ketodan® Foam, 2% Avoid fire, flame and/or smoking during and immediately following ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Medimetriks Pharmaceuticals, Inc. 383 Route 46 West, Fairfield, NJ 07004-2402 www.medimetriks.com - Manufactured by: Padagis, Yeruham, Israel - Made in Israel - IP031-R6 - Rev. Date ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Ketodan® Foam, 2% Important Information: Ketodan® Foam, 2% is for use on the skin only. Do not use Ketodan® Foam, 2% in your eyes, mouth or vagina. What is Ketodan ...
-
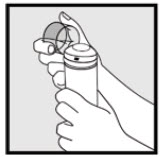
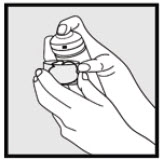
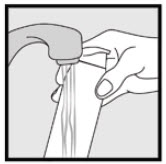
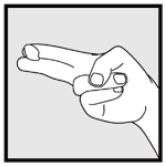
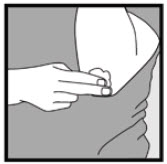
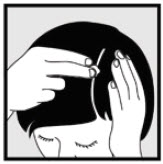
INSTRUCTIONS FOR USEInstructions for Use - Ketodan® Foam, 2% Important Information: Ketodan® Foam, 2% is for use on the skin only. Do not use Ketodan® Foam, 2% in your eyes, mouth or vagina. Step 1: Remove the ...
-
SPL UNCLASSIFIED SECTIONRx Only - Made in Israel - Manufactured by: Padagis, Yeruham, Israel - Manufactured for: Medimetriks Pharmaceuticals, Inc. 383 Route 46 West, Fairfield, NJ 07004-2402 - www.medimetriks.com - IP031-R6 - Rev ...
-
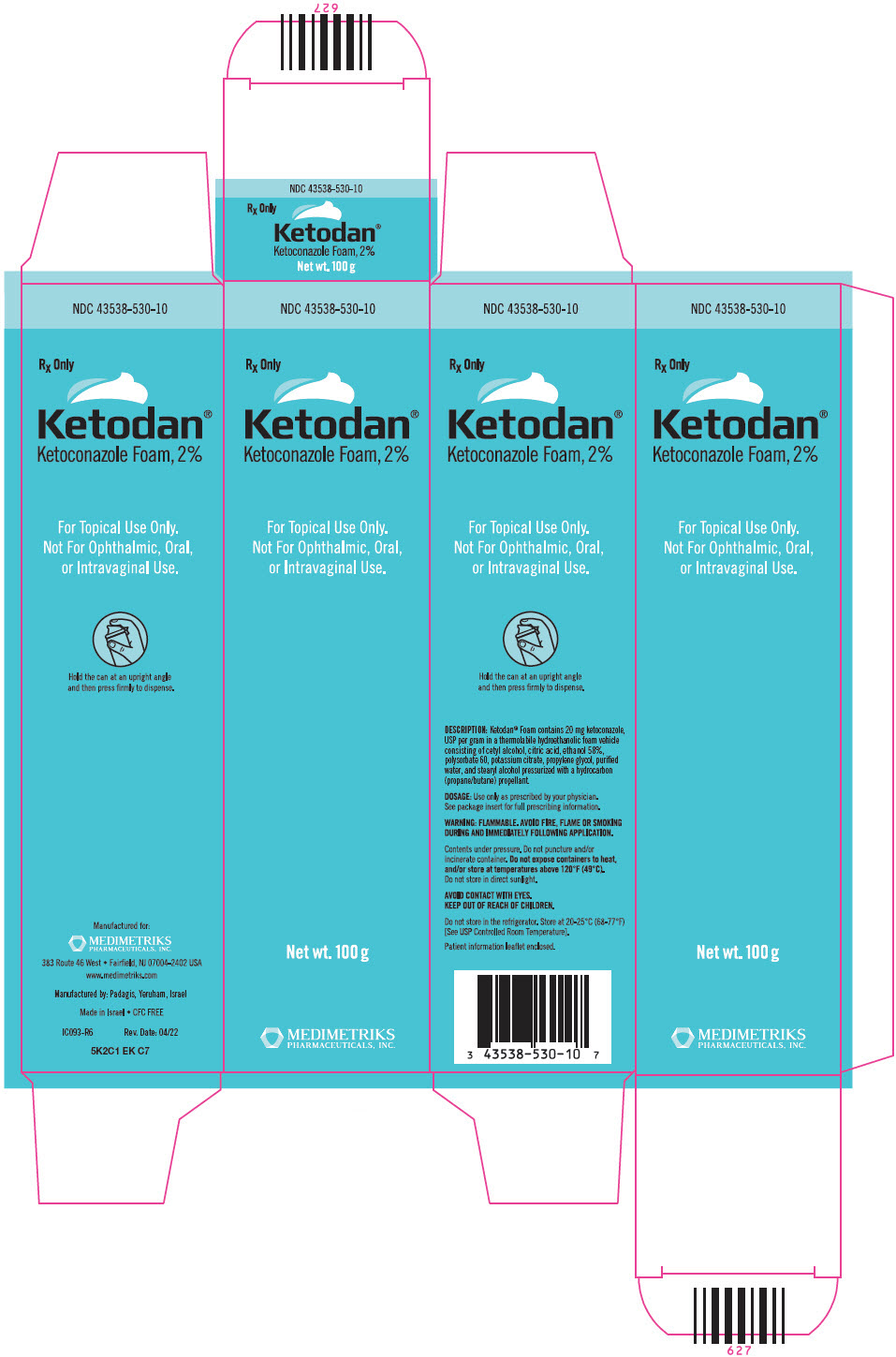
PRINCIPAL DISPLAY PANEL - 100 g Can CartonNDC 43538-530-10 - Rx Only - Ketodan® Ketoconazole Foam, 2% For Topical Use Only. Not For Ophthalmic, Oral, or Intravaginal Use. Net wt. 100 g - MEDIMETRIKS - PHARMACEUTICALS, INC.
-
INGREDIENTS AND APPEARANCEProduct Information