Label: KEPIVANCE- palifermin injection, powder, lyophilized, for solution
- NDC Code(s): 66658-113-01, 66658-113-03, 66658-113-06
- Packager: Swedish Orphan Biovitrum AB (publ)
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Kepivance safely and effectively. See full prescribing information for KEPIVANCE. KEPIVANCE - ®(palifermin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indications - Kepivance is indicated to decrease the incidence and duration of severe oral mucositis in patients with hematologic malignancies receiving myelotoxic therapy in the setting of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage Regimen - The recommended dose of Kepivance is 60 mcg/kg/day, administered as an intravenous bolus injection for 3 consecutive days before and 3 consecutive days after ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 5.16 mg lyophilized powder in single-dose vials.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Potential for Stimulation of Tumor Growth - The safety and efficacy of Kepivance have not been established in patients with non-hematologic malignancies. The effects of Kepivance on ...
-
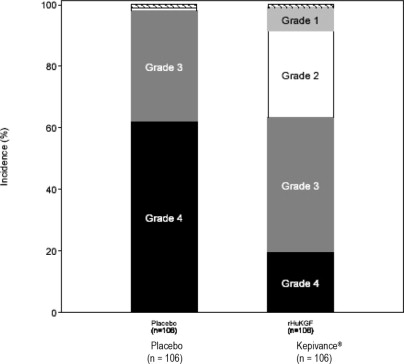
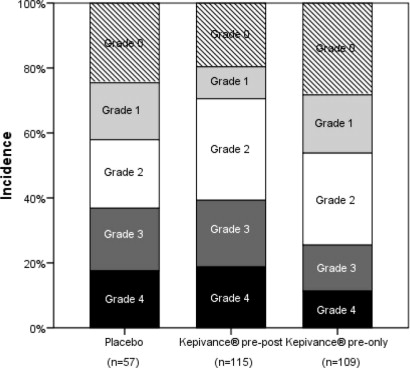
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONSIn vitroand - in vivodata showed that palifermin interacts with unfractionated as well as low molecular weight heparins with no noticeable effect on the pharmacodynamics of either drug. If ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal studies, Kepivance may cause fetal harm when administered to pregnant women. There are no data available on Kepivance use in pregnant ...
-
11 DESCRIPTIONKepivance (palifermin) is a truncated human KGF produced by recominant DNA technology in - E coli. Kepivance is a water soluble, 140 amino acid protein with a molecular weight of 16.3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - KGF is an endogenous protein in the fibroblast growth factor (FGF) family that binds to the KGF receptor. Binding of KGF to its receptor has been reported to result in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Palifermin was not carcinogenic in a 26-week study in rasH2 transgenic mice at intravenous doses of 0.1, 1, or 10 ...
-
14 CLINICAL STUDIES14.1 Autologous transplantation preparative regimens that include total body irradiation - The safety and efficacy of Kepivance in decreasing the incidence and duration of severe oral mucositis ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKepivance is supplied as a lyophilized powder in single-dose vials containing 5.16 mg of palifermin. Kepivance vials are supplied in: a dispensing pack containing 3 vials (NDC 66658-113-03) a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to report the following to healthcare providers: Rashes and reddening of skin - [see Adverse Reactions ( 6.1)] Itchiness - [see Adverse Reactions ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 3 Pack 5.16 mg/1.2 mL Carton Label - sobi - 3 x 5.16 mg/vial Single Use Vials - NDC 66658-113-03 - Kepivance® (palifermin) For Injection - 5.16 mg/vial - For ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 6 Pack 5.16 mg/1.2 mL Carton Label - sobi - 6 x 5.16 mg/vial Single Use Vials - NDC 66658-113-06 - Kepivance® (palifermin) For Injection - 5.16 mg/vial - For ...
-
INGREDIENTS AND APPEARANCEProduct Information