Label: JELMYTO- mitomycin kit
- NDC Code(s): 72493-101-40, 72493-102-20, 72493-103-03
- Packager: UroGen Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use JELMYTO safely and effectively. See full prescribing information for JELMYTO. JELMYTO® (mitomycin) for pyelocalyceal solution ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEJELMYTO® is indicated for the treatment of adult patients with low-grade Upper Tract Urothelial Cancer (LG-UTUC).
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - See the Instructions for Administration provided separately. JELMYTO is for pyelocalyceal use only. JELMYTO is not for intravenous use, topical use ...
-
3 DOSAGE FORMS AND STRENGTHSFor pyelocalyceal solution: A kit containing the following: Two 40 mg (each) single-dose vials of sterile, lyophilized, grey to greyish-purple, cake or powder of mitomycin for pyelocalyceal ...
-
4 CONTRAINDICATIONSJELMYTO is contraindicated in patients with perforation of the bladder or upper urinary tract.
-
5 WARNINGS AND PRECAUTIONS5.1 Ureteric Obstruction - Ureteric obstruction, including ureteral stenosis and hydronephrosis, occurred in patients receiving JELMYTO. In the OLYMPUS study, ureteric obstruction was reported in ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Ureteric Obstruction [see Warnings and Precautions (5.1)] Bone Marrow ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals and mechanism of action, JELMYTO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] ...
-
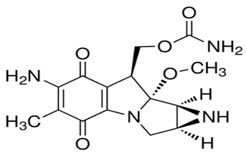
11 DESCRIPTIONMitomycin (also known as mitomycin-C) is an alkylating drug isolated from the broth of Streptomyces. Mitomycin is a blue-violet crystalline powder with a molecular formula of C15H18N4O5, and a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mitomycin inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Adequate long-term studies in animals to evaluate carcinogenic potential from instillation of mitomycin into the pyelocalyceal system ...
-
14 CLINICAL STUDIESThe efficacy of JELMYTO is based on the results of the OLYMPUS study (NCT02793128), an open-label, single-arm, multicenter trial that enrolled 71 patients with treatment-naïve or recurrent ...
-
15 REFERENCES 1. "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - JELMYTO kit – NDC 72493-103-03 - JELMYTO is available in a kit containing the following: Two 40 mg (each) single-dose vials of mitomycin for pyelocalyceal solution supplied as ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Ureteric Obstruction - Inform patients that ureteric obstruction may occur, and ureteral stents or ...
-
SPL UNCLASSIFIED SECTIONDistributed by: UroGen Pharma, Inc. Princeton, NJ 08540 - JELMYTO® and UroGen® are registered trademarks of UroGen Pharma, Ltd. U.S. Patent Nos. 9,040,074 and 9,950,069 - Copyright© 2024 UroGen ...
-
PATIENT PACKAGE INSERTPatient Information - JELMYTO® (jel-MYE-toe) (mitomycin) for pyelocalyceal solution - This Patient Information has been approved by the U.S. Food and Drug Administration.Revised ...
-
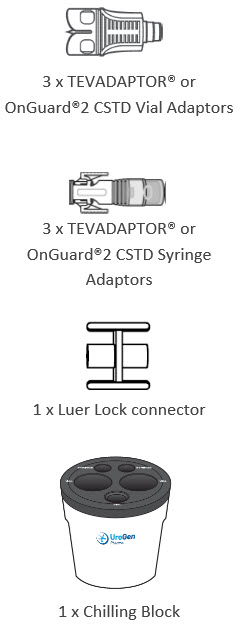
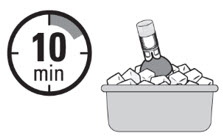
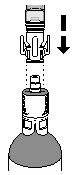
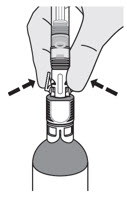
INSTRUCTIONS FOR PHARMACY (IFP) JELMYTO® (jel-MYE-toe) (mitomycin) for pyelocalyceal solutionPurpose of this Instructions for Pharmacy - This Instructions for Pharmacy contains information on how to prepare JELMYTO using pharmacy supplies and a Chilling Block. Intended Use of ...
-
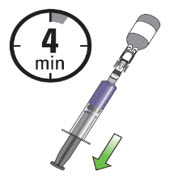
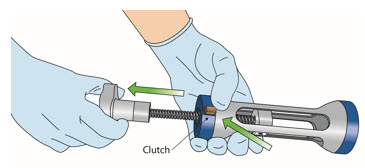
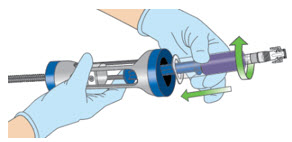
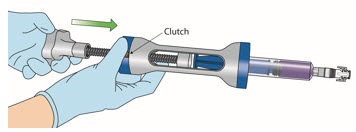
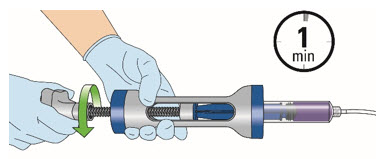
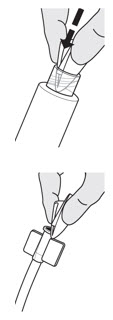
INSTRUCTIONS FOR ADMINISTRATION (IFA) JELMYTO® (jel-MYE-toe) (mitomycin) for pyelocalyceal solutionFor Pyelocalyceal Instillation OnlyRead and follow this Instructions for Administration prior to each JELMYTO instillation. Purpose of this Instructions for Administration - This Instructions for Administration contains ...
-
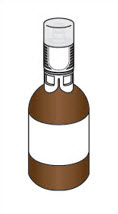
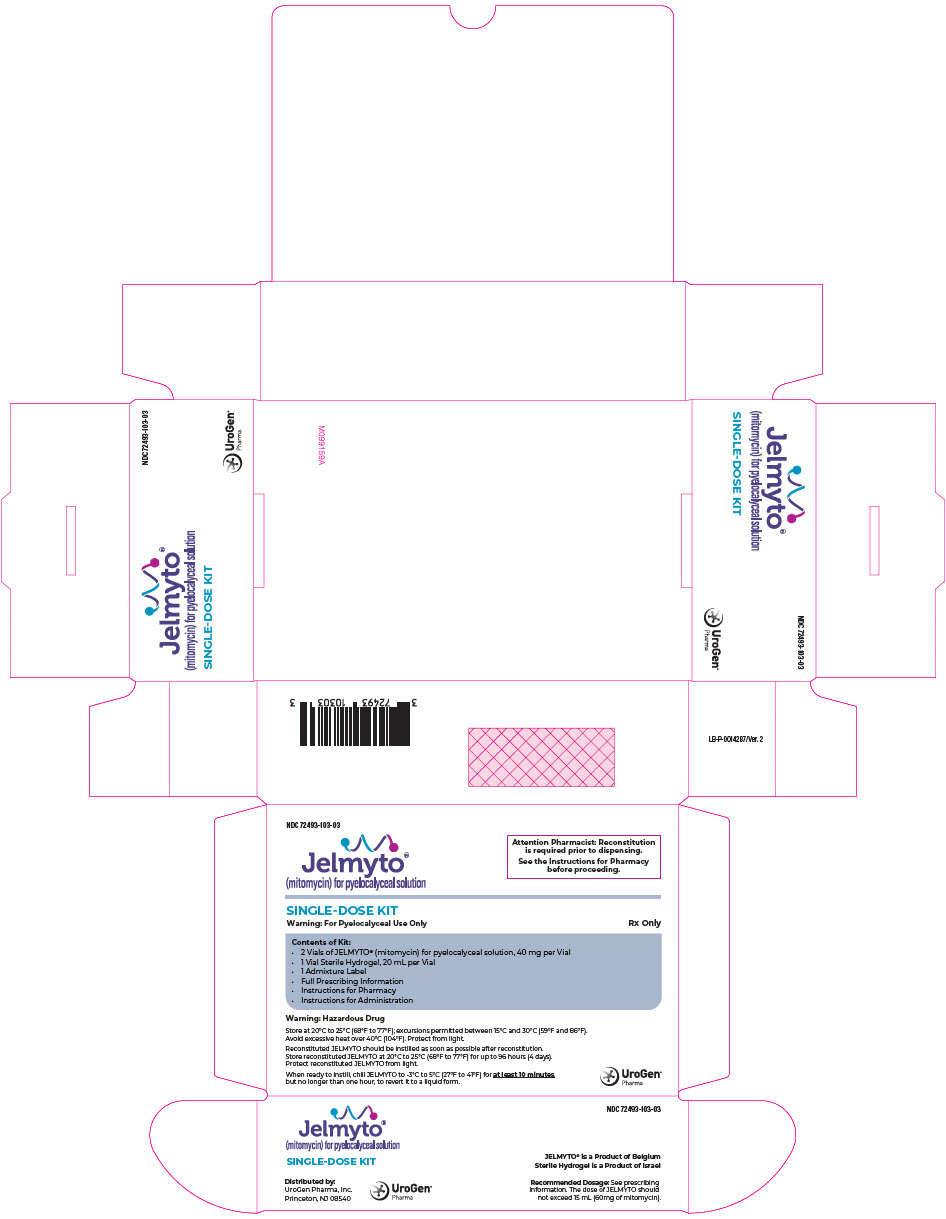
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 72493-103-03 - Jelmyto® (mitomycin) for pyelocalyceal solution - Attention Pharmacist: Reconstitution - is required prior to dispensing. See the Instructions for Pharmacy - before ...
-
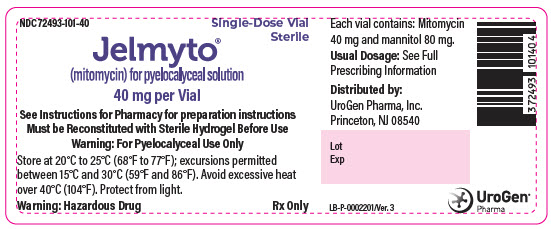
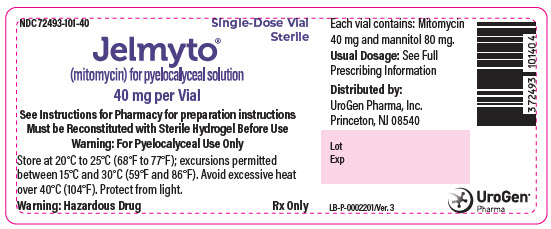
PRINCIPAL DISPLAY PANEL - 40 mg Vial LabelNDC 72493-101-40 - Single-Dose Vial - Sterile - Jelmyto® (mitomycin) for pyelocalyceal solution - 40 mg per Vial - See Instructions for Pharmacy for preparation instructions - Must be Reconstituted with ...
-
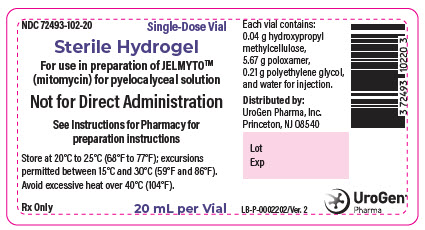
PRINCIPAL DISPLAY PANEL - 20 mL Vial LabelNDC 72493-102-20 - Single-Dose Vial - Sterile Hydrogel - For use in preparation of JELMYTO™ (mitomycin) for pyelocalyceal solution - Not for Direct Administration - See Instructions for Pharmacy ...
-
INGREDIENTS AND APPEARANCEProduct Information