Label: IXEMPRA- ixabepilone kit
- NDC Code(s): 70020-1910-1, 70020-1911-1
- Packager: R-Pharm US Operating, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IXEMPRA - ® safely and effectively. See full prescribing information for IXEMPRA - ®. IXEMPRA - ® Kit (ixabepilone) for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: TOXICITY IN PATIENTS WITH HEPATIC IMPAIRMENT
IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN due to increased risk of toxicity and neutropenia-related death [see Contraindications ( 4) and Warnings and Precautions ( 5.3)].
Close -
1 INDICATIONS AND USAGEIXEMPRA is indicated in combination with capecitabine for the treatment of patients with metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane, or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Premedication - All patients must be premedicated approximately 1 hour before the infusion of IXEMPRA with: An H - 1 antagonist (eg, diphenhydramine 50 mg orally or equivalent) and - An H ...
-
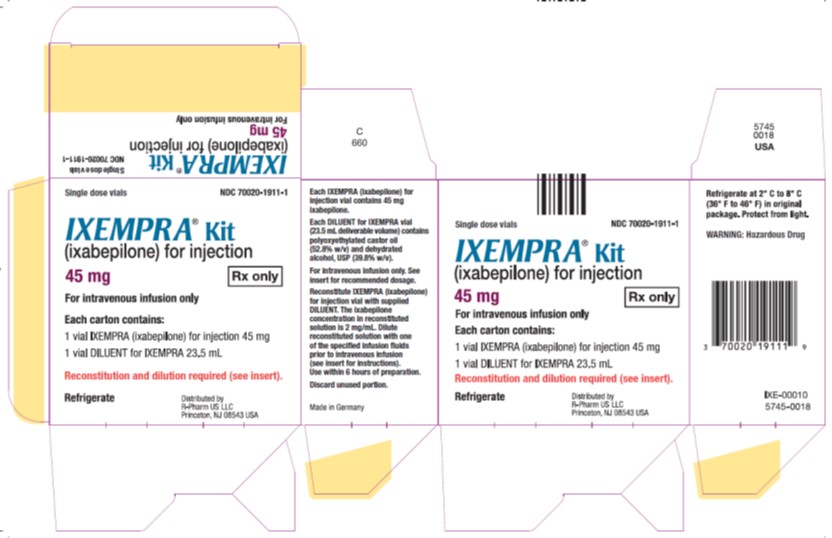
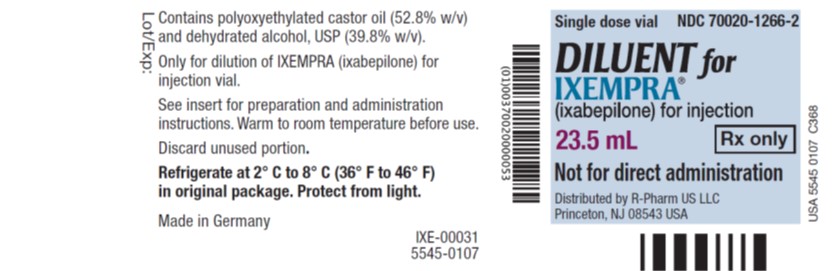
3 DOSAGE FORMS AND STRENGTHSIXEMPRA for injection, 15 mg single-dose vial supplied with DILUENT for IXEMPRA, 8 mL. IXEMPRA for injection, 45 mg single-dose vial supplied with DILUENT for IXEMPRA, 23.5 mL.
-
4 CONTRAINDICATIONSIXEMPRA is contraindicated in patients who have: a neutrophil count <1500 cells/mm - 3 or a platelet count <100,000 cells/mm - 3 [see Warnings and Precautions ( 5.2)]. a history of ...
-
5 WARNINGS AND PRECAUTIONS5.2 Myelosuppression - Severe, life-threatening, or fatal myelosuppression can occur in patients treated with IXEMPRA. Myelosuppression is dose-dependent and primarily manifests as neutropenia ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections. Peripheral neuropathy - [see Warnings and Precautions ( 5.1)] Myelosuppression - [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on IXEMPRA - Strong CYP3A4 Inhibitors - The coadministration of IXEMPRA with a strong CYP3A4 inhibitor increased ixabepilone plasma concentration, which may increase ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals and its mechanism of action, IXEMPRA can cause fetal harm when administered to a pregnant woman - [see Clinical Pharmacology (12.1)] ...
-
10 OVERDOSAGEIn patients who received an overdosage of IXEMPRA of up to 100 mg/m - 2 (approximately 2.5 times the recommended dosage), peripheral neuropathy, fatigue, musculoskeletal pain/myalgia, and ...
-
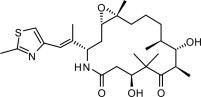
11 DESCRIPTIONIXEMPRA (ixabepilone) is a microtubule inhibitor belonging to a class of antineoplastic agents, the epothilones and their analogs. The epothilones are isolated from the myxobacterium - Sorangium ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Ixabepilone is a semi-synthetic analog of epothilone B. Ixabepilone binds directly to β-tubulin subunits on microtubules, leading to suppression of microtubule ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with ixabepilone have not been conducted. Ixabepilone did not induce mutations in the microbial mutagenesis ...
-
14 CLINICAL STUDIES
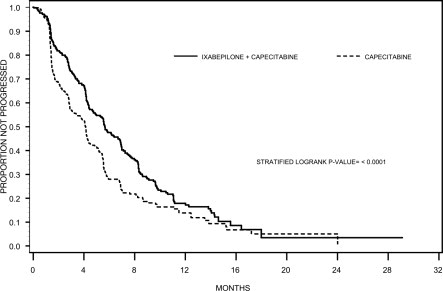
Combination Therapy - In an open-label, multicenter, multinational, randomized trial of 752 patients with metastatic or locally advanced breast cancer, the efficacy and safety of IXEMPRA (40 ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html OSHA
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIXEMPRA is supplied as a - Kit containing one single-dose vial of IXEMPRA - ® (ixabepilone) for injection and one vial of DILUENT for IXEMPRA. NDC 70020-1910-1IXEMPRA - ® Kit ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling - 17.1 Peripheral Neuropathy - Advice patients to report any numbness and tingling of the hands or feet to their healthcare provider ...
-
PATIENT PACKAGE INSERTFDA-Approved Patient Labeling - Patient Information - IXEMPRA® Kit - (ĭk-'sĕm-pră) (ixabepilone) for injection, for intravenous use - What is the most important information I should know ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - Single dose vials NDC 70020-1910-1 - IXEMPRA®Kit - (ixabepilone) for injection - 15 mg Rx only - For intravenous infusion only - Each carton contains - 1 vial ...
-
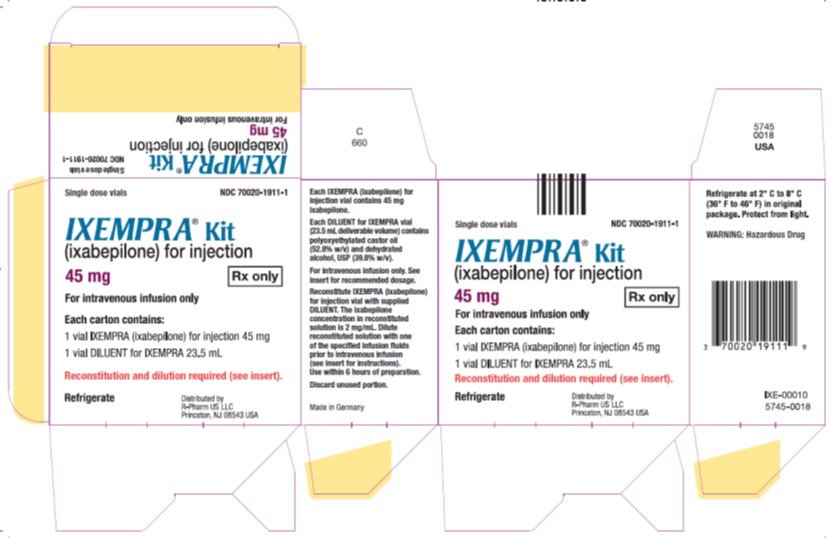
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - Single dose vials NDC 70020-1911-1 - IXEMPRA®Kit - (ixabepilone) for injection - 45 mg Rx only - For intravenous infusion only - Each carton contains - 1 vial ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Vial Label - Single dose vial NDC 70020-1910-2 - IXEMPRA® (ixabepilone) for injection - 15 mg Rx only - For intravenous infusion only - reconstitution and dilution ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Vial Label - Single dose vial NDC 70020-1911-2 - IXEMPRA® (ixabepilone) for injection - 45 mg Rx only - For intravenous infusion only - Reconstitution and dilution ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Vial Label - Single dose vial NDC 70020-1265-1 - DILUENT for - IXEMPRA - ® (ixabepilone) for injection - 8 mL Rx only - Not for direct ...
-
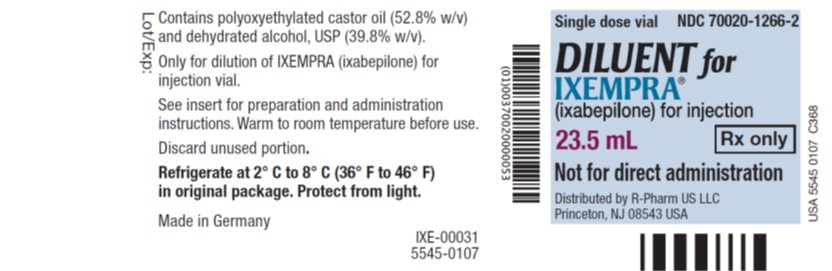
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Vial Label - Single dose vial NDC 70020-1266-2 - DILUENT for - IXEMPRA - ® (ixabepilone) for injection - 23.5 mL Rx only - Not for direct ...
-
INGREDIENTS AND APPEARANCEProduct Information