Label: ISTODAX- romidepsin kit
- NDC Code(s): 59572-961-10, 59572-962-10, 59572-972-02, 59572-973-02, view more
- Packager: Celgene Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ISTODAX safely and effectively. See full prescribing information for ISTODAX. ISTODAX® (romidepsin) for injection, for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEISTODAX is indicated for the treatment of cutaneous T-cell lymphoma (CTCL) in adult patients who have received at least one prior systemic therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - The recommended dosage of romidepsin is 14 mg/m2 administered intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. Cycles should be repeated ...
-
3 DOSAGE FORMS AND STRENGTHSFor Injection: 10 mg of romidepsin as a lyophilized white powder in a single-dose vial for reconstitution and further dilution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Treatment with ISTODAX can cause thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia. Monitor blood counts regularly during treatment with ISTODAX and ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in more detail in other sections of the prescribing information. • Myelosuppression [see Warnings and Precautions (5.1)] • Infections [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Warfarin or Coumarin Derivatives - Prolongation of PT and elevation of INR were observed in a patient receiving ISTODAX concomitantly with warfarin. Monitor PT and INR more frequently in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings from animal studies, ISTODAX can cause embryo-fetal harm when administered to a pregnant woman [see Clinical ...
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage of ISTODAX. Toxicities in a single-dose study in rats or dogs, at intravenous romidepsin doses up to 2.2-fold the recommended ...
-
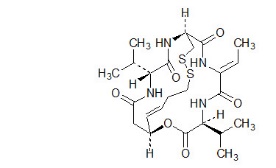
11 DESCRIPTIONRomidepsin, a histone deacetylase (HDAC) inhibitor, is a bicyclic depsipeptide. At room temperature, romidepsin is a white powder and is described chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Romidepsin is a histone deacetylase (HDAC) inhibitor. HDACs catalyze the removal of acetyl groups from acetylated lysine residues in histones, resulting in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with romidepsin. Romidepsin was not mutagenic in vitro in the bacterial reverse ...
-
14 CLINICAL STUDIESISTODAX was evaluated in 2 multicenter, single-arm clinical studies in patients with CTCL (Study 1 [NCT00106431] and Study 2 [NCT00007345]). Overall, 167 patients with CTCL were treated in the ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. [Accessed on 09/11/2018, from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ISTODAX is supplied as a kit including a sterile, lyophilized powder in a 10 mg single-dose vial containing 11 mg of romidepsin, 22 mg of the bulking agent, povidone, USP, and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Low Blood Counts - Advise patients that treatment with ISTODAX can cause low blood counts and that frequent ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ISTODAX (ISS toe dax) (romidepsin) for injection - What is ISTODAX? ISTODAX is a prescription medicine used to treat people with a type of cancer called cutaneous ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 59572-984-01 - ISTODAX® Kit - (romidepsin) for injection - 10 mg/vial - Rx Only - Reconstitution and dilution required. Each kit contains: — One 10 mg single-dose vial of ISTODAX® — One ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Vial LabelNDC 59572-962-10 - ISTODAX® (romidepsin) for injection - Rx Only - 10 mg/vial - FOR INTRAVENOUS USE ONLY. Directions For Use: See accompanying package - insert for complete reconstitution ...
-
PRINCIPAL DISPLAY PANEL - 2.2 mL Vial LabelDILUENT - for ISTODAX® NDC 59572-973-02 - Rx Only - 2.2 mL per single-dose vial - Each vial contains 80% propylene glycol and 20% dehydrated - alcohol. Dosage and Administration: Withdraw 2.2 mL - of Diluent ...
-
INGREDIENTS AND APPEARANCEProduct Information