Label: IONOSOL MB AND DEXTROSE- dextrose monohydrate, sodium lactate, potassium chloride, magnesium chloride, potassium phospha...view full title
- NDC Code(s): 0990-7372-03, 0990-7372-62
- Packager: ICU Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION(MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP) A MAINTENANCE ELECTROLYTE SOLUTION - Flexible Plastic Container - Rx only
-
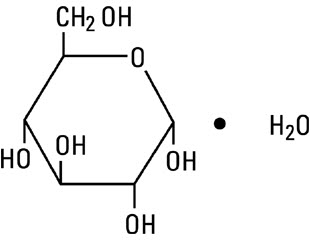
DESCRIPTIONIonosol MB and 5% Dextrose Injection (Multiple Electrolytes and 5% Dextrose Injection Type 1, USP) is a sterile, nonpyrogenic solution designed for intravenous administration. The solution is ...
-
CLINICAL PHARMACOLOGYIonosol MB and 5% Dextrose Injection contains a hypotonic concentration of electrolytes with dextrose. The letters "MB" mean "modified Butler's" solution; the modified solution contains 5 mEq less ...
-
INDICATIONS AND USAGEIonosol MB and 5% Dextrose Injection is indicated for intravenous administration to infants for treatment of dehydration, acidosis, diarrhea, and burns, but only after administration of an initial ...
-
CONTRAINDICATIONSSolutions containing potassium are contraindicated in diseases where high potassium levels may be encountered.
-
WARNINGSSolutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is ...
-
PRECAUTIONSClinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy ...
-
ADVERSE REACTIONSReactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the ...
-
OVERDOSAGEIn the event of overhydration or solute overload during therapy, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
-
DOSAGE AND ADMINISTRATIONThe dose is dependent upon the age, weight and clinical condition of the patient. In infants, Ionosol MB and 5% Dextrose Injection is given only after administration of an initial priming ...
-
HOW SUPPLIEDIonosol MB and 5% Dextrose Injection (Multiple Electrolytes and 5% Dextrose Injection Type 1, USP) is supplied in flexible plastic single-dose containers as follows: NDCFill Volume/Container ...
-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label500 mL - NDC 0990-7372-03 - IONOSOL® MB and - 5% DEXTROSE INJECTION - (MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP) A MAINTENANCE ELECTROLYTE SOLUTION - EACH 100 mL CONTAINS DEXTROSE ...
-
PRINCIPAL DISPLAY PANEL - Overwrap LabelTO OPEN TEAR AT NOTCH - 2 - HDPE - DO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING - THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER FIRMLY. IF LEAKS ARE FOUND, DISCARD ...
-
INGREDIENTS AND APPEARANCEProduct Information