Label: INVELTYS- loteprednol etabonate suspension
- NDC Code(s): 71571-121-20, 71571-121-28
- Packager: ALCON LABORATORIES, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INVELTYS® safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol etabonate ophthalmic ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEINVELTYS is a corticosteroid indicated for the treatment of post-operative inflammation and pain following ocular surgery.
-
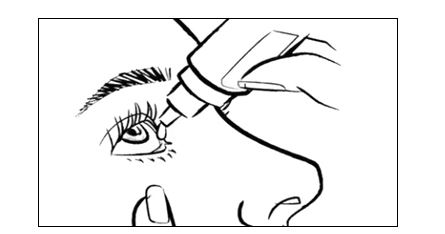
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - Instill one to two drops of INVELTYS into the affected eye twice daily beginning the day after surgery and continuing throughout the first 2 weeks of the post-operative ...
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic suspension containing 10 mg/mL of loteprednol etabonate.
-
4 CONTRAINDICATIONSINVELTYS is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intraocular Pressure (IOP) Increase - Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, as well as defects in visual acuity and fields of vision ...
-
6 ADVERSE REACTIONSAdverse reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with infrequent optic nerve damage, visual acuity and field defects, posterior ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - INVELTYS is not absorbed systemically following topical ophthalmic administration and maternal use is not expected to result in fetal exposure to the ...
-
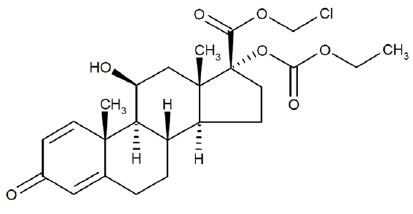
11 DESCRIPTIONLoteprednol etabonate is a corticosteroid. Its chemical name is chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylate. Its molecular formula is C24H31ClO7 and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been conducted to evaluate the carcinogenic potential of loteprednol etabonate. Loteprednol etabonate ...
-
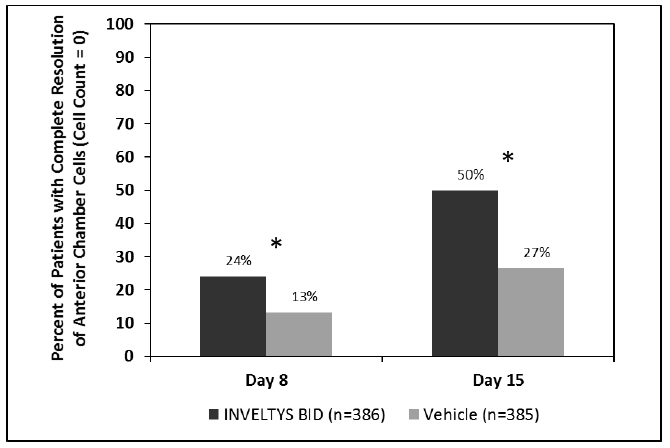
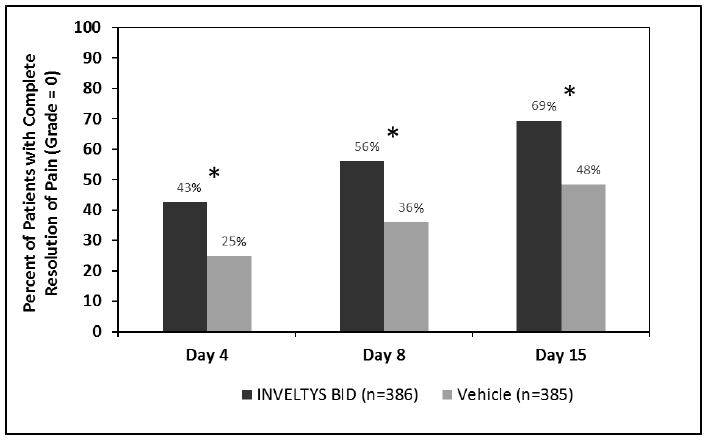
14 CLINICAL STUDIESClinical efficacy was evaluated in 2 multi-centered, randomized, double-masked, placebo-controlled trials in which patients with an anterior cell grade greater than or equal to "2" (a cell count ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGINVELTYS (loteprednol etabonate ophthalmic suspension) 1% is a sterile ophthalmic suspension. It is supplied in a white low-density polyethylene plastic dropper bottle with a controlled-drop ...
-
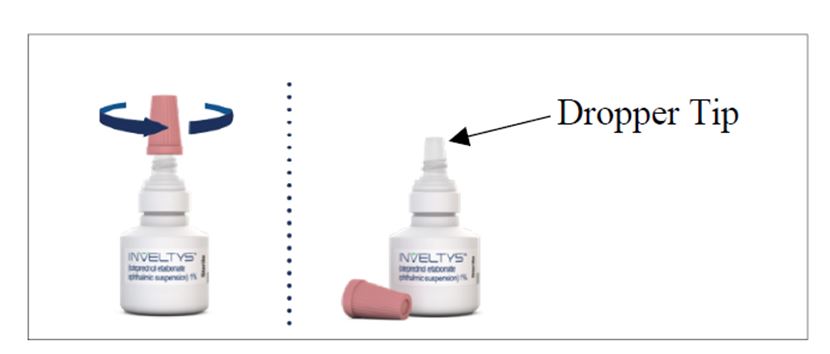
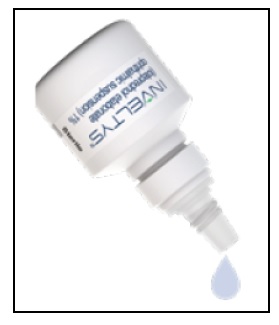
17 PATIENT COUNSELING INFORMATIONAdministration - Instruct the patient to shake the bottle for one to two seconds before using. If the patient is using other eye drops in addition to INVELTYS, advise the patient to wait at ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Alcon Laboratories, Inc. Fort Worth, TX 76134 USA - U.S. Pat.: www.alconpatents.com - © 2023 Alcon Inc. Alcon
-
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - INVELTYS [in-vel-tis] (loteprednol etabonate ophthalmic suspension) 1% for topical ophthalmic use - This Instructions for Use contains information on how to ...
-
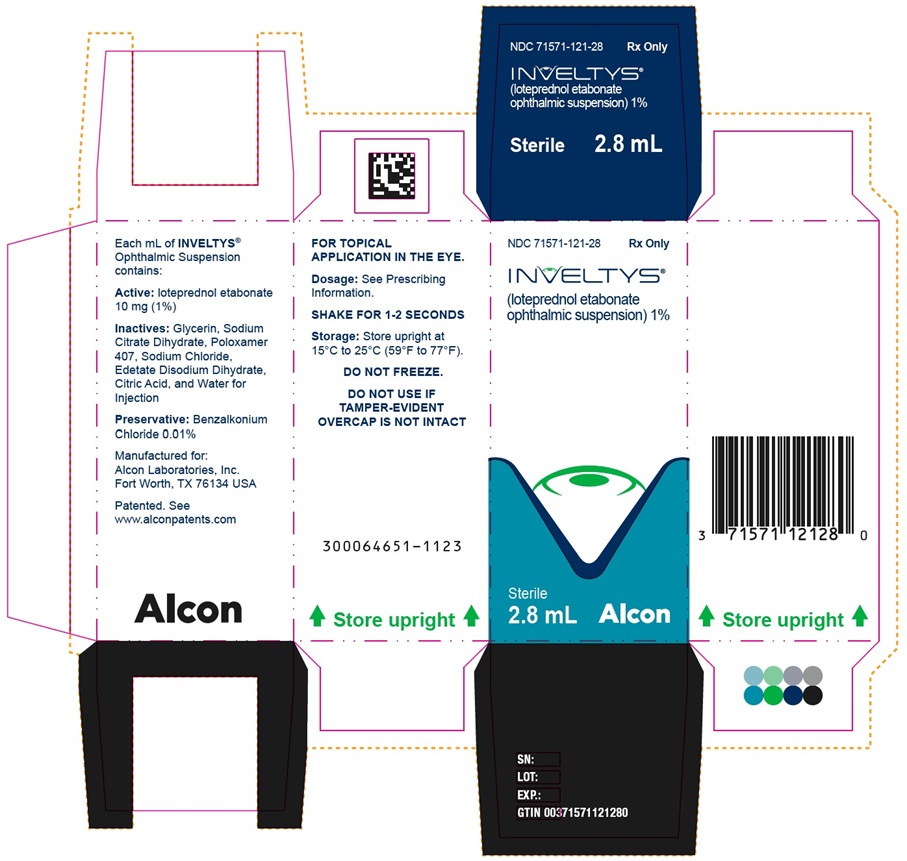
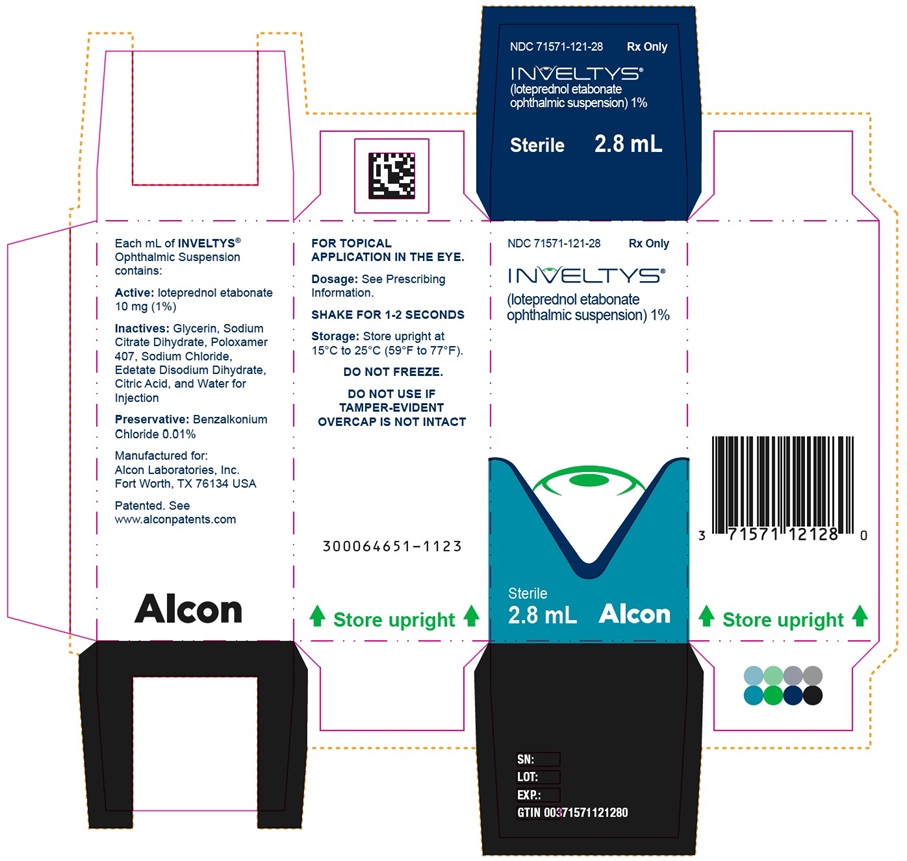
PRINCIPAL DISPLAY PANEL - 2.8 mL Bottle CartonNDC 71571-121-28 - Rx Only - INVELTYS® (loteprednol etabonate ophthalmic suspension)1% Sterile - 2.8 mL - kala® PHARMACEUTICALS - NDC 71571-121-28 - Rx Only - INVELTYS® (loteprednol ...
-
INGREDIENTS AND APPEARANCEProduct Information