Label: INVANZ- ertapenem sodium injection, powder, lyophilized, for solution
- NDC Code(s): 0006-3843-71, 0006-3845-01, 0006-3845-71
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INVANZ safely and effectively. See full prescribing information for INVANZ. INVANZ® (ertapenem for injection), for intravenous or ...These highlights do not include all the information needed to use INVANZ safely and effectively. See full prescribing information for INVANZ.
INVANZ® (ertapenem for injection), for intravenous or intramuscular use
Initial U.S. Approval: 2001INDICATIONS AND USAGE
INVANZ is a penem antibacterial indicated in adult patients and pediatric patients (3 months of age and older) for the treatment of the following moderate to severe infections caused by susceptible bacteria:
- Complicated intra-abdominal infections. (1.1)
- Complicated skin and skin structure infections, including diabetic foot infections without osteomyelitis. (1.2)
- Community-acquired pneumonia. (1.3)
- Complicated urinary tract infections including pyelonephritis. (1.4)
- Acute pelvic infections including postpartum endomyometritis, septic abortion and post-surgical gynecologic infections. (1.5)
INVANZ is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. (1.6)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of INVANZ and other antibacterial drugs, INVANZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.7)
DOSAGE AND ADMINISTRATION
Do not mix or co-infuse INVANZ with other medications. Do not use diluents containing dextrose (α–D–glucose). (2.1)
INVANZ should be infused over 30 minutes in both the Treatment and Prophylactic regimens. (2.1)
Dosing considerations should be made in adults with advanced or end-stage renal impairment and those on hemodialysis. (2.4, 2.5)
Treatment regimen:
- Adults and pediatric patients 13 years of age and older. The dosage should be 1 gram once a day intravenously or intramuscularly. (2.2)
- Patients 3 months to 12 years of age should be administered 15 mg/kg twice daily (not to exceed 1 g/day intravenously or intramuscularly). (2.2)
- Intravenous infusion may be administered in adults and pediatrics for up to 14 days or intramuscular injection for up to 7 days. (2.1)
Prophylaxis regimen for adults:
- 1 gram single dose given 1 hour prior to elective colorectal surgery. (2.3)
DOSAGE FORMS AND STRENGTHS
- For injection: Single-dose vial 1 gram. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serious hypersensitivity (anaphylactic) reactions have been reported in patients receiving β-lactams. (5.1)
- Seizures and other central nervous system adverse experiences have been reported during treatment. (5.2)
- Co-administration of INVANZ with valproic acid or divalproex sodium reduces the serum concentration of valproic acid potentially increasing the risk of breakthrough seizures. (5.3)
- Clostridioides difficile-associated diarrhea (ranging from mild diarrhea to fatal colitis): Evaluate if diarrhea occurs. (5.4)
- Caution should be taken when administering INVANZ intramuscularly to avoid inadvertent injection into a blood vessel. (5.5)
ADVERSE REACTIONS
Adults:
The most common adverse reactions (≥5%) in patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea, nausea, headache and infused vein complication. (6.1)
In the prophylaxis indication the overall adverse experience profile was generally comparable to that observed for ertapenem in other clinical trials. (6.1)
Pediatrics:
Adverse reactions in this population were comparable to adults. The most common adverse reactions (≥5%) in pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea, vomiting and infusion site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .DRUG INTERACTIONS
- Co-administration with probenecid inhibits the renal excretion of ertapenem and is therefore not recommended. (7.1)
- The concomitant use of ertapenem and valproic acid/divalproex sodium is generally not recommended. Anti-bacterials other than carbapenems should be considered to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium. (5.2, 7.2)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Complicated Intra-Abdominal Infections
1.2 Complicated Skin and Skin Structure Infections, Including Diabetic Foot Infections without Osteomyelitis
1.3 Community Acquired Pneumonia
1.4 Complicated Urinary Tract Infections Including Pyelonephritis
1.5 Acute Pelvic Infections Including Postpartum Endomyometritis, Septic Abortion and Post-Surgical Gynecologic Infections
1.6 Prophylaxis of Surgical Site Infection Following Elective Colorectal Surgery
1.7 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Use in All Patients
2.2 Treatment Regimen
2.3 Prophylactic Regimen in Adults
2.4 Patients with Renal Impairment
2.5 Patients on Hemodialysis
2.6 Patients with Hepatic Impairment
2.7 Preparation and Reconstitution for Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Seizure Potential
5.3 Interaction with Valproic Acid
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
5.5 Caution with Intramuscular Administration
5.6 Development of Drug-Resistant Bacteria
5.7 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
6.3 Adverse Laboratory Changes in Clinical Trials
7 DRUG INTERACTIONS
7.1 Probenecid
7.2 Valproic Acid
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adults
14.2 Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
17.1 Instructions for Patients
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Complicated Intra-Abdominal Infections - INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with complicated intra-abdominal infections ...
1.1 Complicated Intra-Abdominal Infections
INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with complicated intra-abdominal infections due to Escherichia coli, Clostridium clostridioforme, Eubacterium lentum, Peptostreptococcus species, Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, or Bacteroides uniformis.
1.2 Complicated Skin and Skin Structure Infections, Including Diabetic Foot Infections without Osteomyelitis
INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with complicated skin and skin structure infections, including diabetic foot infections without osteomyelitis due to Staphylococcus aureus (methicillin susceptible isolates only), Streptococcus agalactiae, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Bacteroides fragilis, Peptostreptococcus species, Porphyromonas asaccharolytica, or Prevotella bivia. INVANZ has not been studied in diabetic foot infections with concomitant osteomyelitis [see Clinical Studies (14)].
1.3 Community Acquired Pneumonia
INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with community acquired pneumonia due to Streptococcus pneumoniae (penicillin susceptible isolates only) including cases with concurrent bacteremia, Haemophilus influenzae (beta-lactamase negative isolates only), or Moraxella catarrhalis.
1.4 Complicated Urinary Tract Infections Including Pyelonephritis
INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with complicated urinary tract infections including pyelonephritis due to Escherichia coli, including cases with concurrent bacteremia, or Klebsiella pneumoniae.
1.5 Acute Pelvic Infections Including Postpartum Endomyometritis, Septic Abortion and Post-Surgical Gynecologic Infections
INVANZ is indicated for the treatment of adult patients and pediatric patients (3 months of age and older) with acute pelvic infections including postpartum endomyometritis, septic abortion and post-surgical gynecological infections due to Streptococcus agalactiae, Escherichia coli, Bacteroides fragilis, Porphyromonas asaccharolytica, Peptostreptococcus species, or Prevotella bivia.
1.6 Prophylaxis of Surgical Site Infection Following Elective Colorectal Surgery
INVANZ is indicated in adults for the prevention of surgical site infection following elective colorectal surgery.
Close1.7 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of INVANZ and other antibacterial drugs, INVANZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Instructions for Use in All Patients - For Intravenous or Intramuscular Use - DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE ...
2.1 Instructions for Use in All Patients
For Intravenous or Intramuscular Use
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ may be administered by intravenous infusion for up to 14 days or intramuscular injection for up to 7 days. When administered intravenously, INVANZ should be infused over a period of 30 minutes. Intramuscular administration of INVANZ may be used as an alternative to intravenous administration in the treatment of those infections for which intramuscular therapy is appropriate.
2.2 Treatment Regimen
13 years of age and older
The dose of INVANZ in patients 13 years of age and older is 1 gram (g) given once a day [see Clinical Pharmacology (12.3)].
3 months to 12 years of age
The dose of INVANZ in patients 3 months to 12 years of age is 15 mg/kg twice daily (not to exceed 1 g/day).
Table 1 presents treatment guidelines for INVANZ.
Table 1 Treatment Guidelines for Adults and Pediatric Patients With Normal Renal Function* and Body Weight Infection† Daily Dose
(IV or IM)
Adults and Pediatric Patients 13 years of age and olderDaily Dose
(IV or IM)
Pediatric Patients 3 months to 12 years of ageRecommended Duration of Total Antimicrobial Treatment - *
- defined as creatinine clearance >90 mL/min/1.73 m2
- †
- due to the designated pathogens [see Indications and Usage (1)]
- ‡
- not to exceed 1 g/day
- §
- INVANZ has not been studied in diabetic foot infections with concomitant osteomyelitis [see Clinical Studies (14.1)].
- ¶
- adult patients with diabetic foot infections received up to 28 days of treatment (parenteral or parenteral plus oral switch therapy)
- #
- duration includes a possible switch to an appropriate oral therapy, after at least 3 days of parenteral therapy, once clinical improvement has been demonstrated.
Complicated intra-abdominal infections 1 g 15 mg/kg
twice daily‡5 to 14 days
Complicated skin and skin structure infections, including diabetic foot infections§
1 g
15 mg/kg
twice daily‡
7 to 14 days¶
Community acquired pneumonia
1 g
15 mg/kg
twice daily‡
10 to 14 days#
Complicated urinary tract infections, including pyelonephritis
1 g
15 mg/kg
twice daily‡
10 to 14 days#
Acute pelvic infections including postpartum endomyometritis, septic abortion and post-surgical gynecologic infections
1 g
15 mg/kg
twice daily‡
3 to 10 days2.3 Prophylactic Regimen in Adults
Table 2 presents prophylaxis guidelines for INVANZ.
Table 2 Prophylaxis Guidelines for Adults Indication Daily Dose
(IV)
AdultsRecommended Duration of Total Antimicrobial Treatment Prophylaxis of surgical site infection following elective colorectal surgery 1 g Single intravenous dose given 1 hour prior to surgical incision 2.4 Patients with Renal Impairment
INVANZ may be used for the treatment of infections in adult patients with renal impairment. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with severe renal impairment (creatinine clearance ≤30 mL/min/1.73 m2) and end-stage renal disease (creatinine clearance ≤10 mL/min/1.73 m2) should receive 500 mg daily. A supplementary dose of 150 mg is recommended if ertapenem is administered within 6 hours prior to hemodialysis. There are no data in pediatric patients with renal impairment.
2.5 Patients on Hemodialysis
When adult patients on hemodialysis are given the recommended daily dose of 500 mg of INVANZ within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If INVANZ is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis.
When only the serum creatinine is available, the following formula1 may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function.
Males: (weight in kg) x (140-age in years)
(72) x serum creatinine (mg/100 mL)Females: (0.85) x (value calculated for males)
- 1
-
Cockcroft and Gault equation: Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976
2.6 Patients with Hepatic Impairment
No dose adjustment recommendations can be made in patients with hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Close2.7 Preparation and Reconstitution for Administration
Vials
Adults and pediatric patients 13 years of age and older
Preparation for intravenous administration:
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.
- Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection, using a syringe equipped with a 21-gauge or smaller diameter needle. NOTE: Use with a needleless IV system is not recommended.
- Shake well to dissolve and immediately transfer contents of the reconstituted vial to 50 mL of 0.9% Sodium Chloride Injection.
- Complete the infusion within 6 hours of reconstitution.
Preparation for intramuscular administration:
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.
- Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% lidocaine HCl injection2 (without epinephrine). Shake vial thoroughly to form solution.
- Immediately withdraw the contents of the vial and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh).
- The reconstituted IM solution should be used within 1 hour after preparation. NOTE: THE RECONSTITUTED SOLUTION SHOULD NOT BE ADMINISTERED INTRAVENOUSLY.
Pediatric patients 3 months to 12 years of age
Preparation for intravenous administration:
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.
- Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection, using a syringe equipped with a 21-gauge or smaller diameter needle. NOTE: Use with a needleless IV system is not recommended.
- Shake well to dissolve and immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and dilute in 0.9% Sodium Chloride Injection to a final concentration of 20 mg/mL or less. Discard vial with unused portion of INVANZ reconstituted solution.
- Complete the infusion within 6 hours of reconstitution.
Preparation for intramuscular administration:
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.
- Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% lidocaine HCl injection (without epinephrine). Shake vial thoroughly to form solution.
- Immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh). Discard vial with unused portion of INVANZ reconstituted solution.
- The reconstituted IM solution should be used within 1 hour after preparation. NOTE: THE RECONSTITUTED SOLUTION SHOULD NOT BE ADMINISTERED INTRAVENOUSLY.
- 2
-
Refer to the prescribing information for lidocaine HCl.
Storage
When prepared with the diluent, INVANZ (Ertapenem for Injection) maintains satisfactory potency for 6 hours at room temperature (25°C) or for 24 hours under refrigeration (5°C) and used within 4 hours after removal from refrigeration. Solutions of INVANZ should not be frozen.
Before administering, see accompanying package circular for INVANZ (Ertapenem for Injection).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to use, whenever solution and container permit. Solutions of INVANZ range from colorless to pale yellow. Variations of color within this range do not affect the potency of the product.
-
3 DOSAGE FORMS AND STRENGTHSFor Injection: Vials - INVANZ is a sterile lyophilized powder in a single-dose vial containing 1 g ertapenem equivalent to 1.046 g ertapenem sodium for intravenous infusion or for intramuscular ...
For Injection: Vials
INVANZ is a sterile lyophilized powder in a single-dose vial containing 1 g ertapenem equivalent to 1.046 g ertapenem sodium for intravenous infusion or for intramuscular injection after reconstitution.
Close -
4 CONTRAINDICATIONSINVANZ is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions ...
- INVANZ is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams.
- Due to the use of lidocaine HCl as a diluent, INVANZ administered intramuscularly is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide type.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with beta-lactams. These reactions are ...
5.1 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with beta-lactams. These reactions are more likely to occur in individuals with a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe hypersensitivity reactions when treated with another beta-lactam. Before initiating therapy with INVANZ, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, other beta-lactams and other allergens. If an allergic reaction to INVANZ occurs, discontinue the drug immediately. Serious anaphylactic reactions require immediate emergency treatment as clinically indicated.
5.2 Seizure Potential
Seizures and other central nervous system (CNS) adverse experiences have been reported during treatment with INVANZ [see Adverse Reactions (6.1)]. During clinical investigations in adult patients treated with INVANZ (1 g once a day), seizures, irrespective of drug relationship, occurred in 0.5% of patients during study therapy plus 14-day follow-up period [see Adverse Reactions (6.1)]. These experiences have occurred most commonly in patients with CNS disorders (e.g., brain lesions or history of seizures) and/or compromised renal function. Close adherence to the recommended dosage regimen is urged, especially in patients with known factors that predispose to convulsive activity. Anticonvulsant therapy should be continued in patients with known seizure disorders. If focal tremors, myoclonus, or seizures occur, patients should be evaluated neurologically, placed on anticonvulsant therapy if not already instituted, and the dosage of INVANZ re-examined to determine whether it should be decreased or discontinued.
5.3 Interaction with Valproic Acid
Case reports in the literature have shown that co-administration of carbapenems, including ertapenem, to patients receiving valproic acid or divalproex sodium results in a reduction in valproic acid concentrations. The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures. Increasing the dose of valproic acid or divalproex sodium may not be sufficient to overcome this interaction. The concomitant use of ertapenem and valproic acid/divalproex sodium is generally not recommended. Anti-bacterials other than carbapenems should be considered to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium. If administration of INVANZ is necessary, supplemental anti-convulsant therapy should be considered [see Drug Interactions (7.2)].
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
CDAD has been reported with use of nearly all antibacterial agents, including ertapenem, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of Clostridioides difficile.
Clostridioides difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of Clostridioides difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against Clostridioides difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of Clostridioides difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Caution with Intramuscular Administration
Caution should be taken when administering INVANZ intramuscularly to avoid inadvertent injection into a blood vessel [see Dosage and Administration (2.7)].
5.6 Development of Drug-Resistant Bacteria
As with other antibiotics, prolonged use of INVANZ may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient's condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Prescribing INVANZ in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Close5.7 Laboratory Tests
While INVANZ possesses toxicity similar to the beta-lactam group of antibiotics, periodic assessment of organ system function, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
-
6 ADVERSE REACTIONSThe following are described in greater detail in the Warnings and Precautions section. Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Seizure Potential [see Warnings and ...
The following are described in greater detail in the Warnings and Precautions section.
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Seizure Potential [see Warnings and Precautions (5.2)]
- Interaction with Valproic Acid [see Warnings and Precautions (5.3)]
- Clostridioides difficile-Associated Diarrhea (CDAD) [see Warnings and Precautions (5.4)]
- Caution with Intramuscular Administration [see Warnings and Precautions (5.5)]
- Development of Drug-Resistant Bacteria [see Warnings and Precautions (5.6)]
- Laboratory Tests [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 1954 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [see Clinical Studies (14)]. Most adverse experiences reported in these clinical trials were described as mild to moderate in severity. INVANZ was discontinued due to adverse experiences in 4.7% of patients. Table 3 shows the incidence of adverse experiences reported in ≥2.0% of patients in these trials. The most common drug-related adverse experiences in patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (5.5%), infused vein complication (3.7%), nausea (3.1%), headache (2.2%), and vaginitis in females (2.1%).
Table 3 Incidence (%) of Adverse Experiences Reported During Study Therapy Plus 14-Day Follow-Up in ≥2.0% of Adult Patients Treated With INVANZ in Clinical Trials INVANZ*
1 g dailyPiperacillin/ Tazobactam*
3.375 g q6hINVANZ†
1 g dailyCeftriaxone†
1 or 2 g dailyAdverse Events (N=802) (N=774) (N=1152) (N=942) - *
- Includes Phase IIb/III Complicated intra-abdominal infections, Complicated skin and skin structure infections and Acute pelvic infections trials
- †
- Includes Phase IIb/III Community acquired pneumonia and Complicated urinary tract infections, and Phase IIa trials
- ‡
- Includes agitation, confusion, disorientation, decreased mental acuity, changed mental status, somnolence, stupor
Local: Infused vein complication 7.1 7.9 5.4 6.7 Systemic: Death 2.5 1.6 1.3 1.6 Edema/swelling 3.4 2.5 2.9 3.3 Fever 5.0 6.6 2.3 3.4 Abdominal pain 3.6 4.8 4.3 3.9 Hypotension 2.0 1.4 1.0 1.2 Constipation 4.0 5.4 3.3 3.1 Diarrhea 10.3 12.1 9.2 9.8 Nausea 8.5 8.7 6.4 7.4 Vomiting 3.7 5.3 4.0 4.0 Altered mental status‡ 5.1 3.4 3.3 2.5 Dizziness 2.1 3.0 1.5 2.1 Headache 5.6 5.4 6.8 6.9 Insomnia 3.2 5.2 3.0 4.1 Dyspnea 2.6 1.8 1.0 2.4 Pruritus 2.0 2.6 1.0 1.9 Rash 2.5 3.1 2.3 1.5 Vaginitis 1.4 1.0 3.3 3.7 In patients treated for complicated intra-abdominal infections, death occurred in 4.7% (15/316) of patients receiving INVANZ and 2.6% (8/307) of patients receiving comparator drug. These deaths occurred in patients with significant co-morbidity and/or severe baseline infections. Deaths were considered unrelated to study drugs by investigators.
In clinical trials, seizure was reported during study therapy plus 14-day follow-up period in 0.5% of patients treated with INVANZ, 0.3% of patients treated with piperacillin/tazobactam and 0% of patients treated with ceftriaxone [see Warnings and Precautions (5.2)].
Additional adverse experiences that were reported with INVANZ with an incidence >0.1% within each body system are listed below
Body as a Whole: abdominal distention, pain, chills, septicemia, septic shock, dehydration, gout, malaise, asthenia/fatigue, necrosis, candidiasis, weight loss, facial edema, injection site induration, injection site pain, extravasation, phlebitis/thrombophlebitis, flank pain, syncope
Cardiovascular System: heart failure, hematoma, chest pain, hypertension, tachycardia, cardiac arrest, bradycardia, arrhythmia, atrial fibrillation, heart murmur, ventricular tachycardia, asystole, subdural hemorrhage
Digestive System: acid regurgitation, oral candidiasis, dyspepsia, gastrointestinal hemorrhage, anorexia, flatulence, C. difficile-associated diarrhea, stomatitis, dysphagia, hemorrhoids, ileus, cholelithiasis, duodenitis, esophagitis, gastritis, jaundice, mouth ulcer, pancreatitis, pyloric stenosis
Musculoskeletal System: leg pain
Nervous System & Psychiatric: anxiety, nervousness, seizure [see Warnings and Precautions (5.2)], tremor, depression, hypesthesia, spasm, paresthesia, aggressive behavior, vertigo
Respiratory System: cough, pharyngitis, rales/rhonchi, respiratory distress, pleural effusion, hypoxemia, bronchoconstriction, pharyngeal discomfort, epistaxis, pleuritic pain, asthma, hemoptysis, hiccups, voice disturbance
Skin & Skin Appendage: erythema, sweating, dermatitis, desquamation, flushing, urticaria
Special Senses: taste perversion
Urogenital System: renal impairment, oliguria/anuria, vaginal pruritus, hematuria, urinary retention, bladder dysfunction, vaginal candidiasis, vulvovaginitis.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post-surgery, the overall adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. Table 4 shows the incidence of adverse experiences other than those previously described above for INVANZ that were reported regardless of causality in ≥2.0% of patients in this trial.
Table 4 Incidence (%) of Adverse Experiences Reported During Study Therapy Plus 14-Day Follow-Up in ≥2.0% of Adult Patients Treated With INVANZ for Prophylaxis of Surgical Site Infections Following Elective Colorectal Surgery Adverse Events INVANZ
1 g
(N = 476)Cefotetan
2 g
(N = 476)Anemia 5.7 6.9 Small intestinal obstruction 2.1 1.9 Pneumonia 2.1 4.0 Postoperative infection 2.3 4.0 Urinary tract infection 3.8 5.5 Wound infection 6.5 12.4 Wound complication 2.9 2.3 Atelectasis 3.4 1.9 Additional adverse experiences that were reported in this prophylaxis trial with INVANZ, regardless of causality, with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders: C. difficile infection or colitis, dry mouth, hematochezia
General Disorders and Administration Site Condition: crepitations
Infections and Infestations: cellulitis, abdominal abscess, fungal rash, pelvic abscess
Injury, Poisoning and Procedural Complications: incision site complication, incision site hemorrhage, intestinal stoma complication, anastomotic leak, seroma, wound dehiscence, wound secretion
Musculoskeletal and Connective Tissue Disorders: muscle spasms
Nervous System Disorders: cerebrovascular accident
Renal and Urinary Disorders: dysuria, pollakiuria
Respiratory, Thoracic and Mediastinal Disorders: crackles lung, lung infiltration, pulmonary congestion, pulmonary embolism, wheezing.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 384 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [see Clinical Studies (14)]. The overall adverse experience profile in pediatric patients is comparable to that in adult patients. Table 5 shows the incidence of adverse experiences reported in ≥2.0% of pediatric patients in clinical trials. The most common drug-related adverse experiences in pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (6.5%), infusion site pain (5.5%), infusion site erythema (2.6%), vomiting (2.1%).
Table 5 Incidence (%) of Adverse Experiences Reported During Study Therapy Plus 14-Day Follow-Up in ≥2.0% of Pediatric Patients Treated With INVANZ in Clinical Trials INVANZ*,† Ceftriaxone* Ticarcillin/ Clavulanate† Adverse Events (N=384) (N=100) (N=24) - *
- Includes Phase IIb Complicated skin and skin structure infections, Community acquired pneumonia and Complicated urinary tract infections trials in which patients 3 months to 12 years of age received INVANZ 15 mg/kg IV twice daily up to a maximum of 1 g or ceftriaxone 50 mg/kg/day IV in two divided doses up to a maximum of 2 g, and patients 13 to 17 years of age received INVANZ 1 g IV daily or ceftriaxone 50 mg/kg/day IV in a single daily dose.
- †
- Includes Phase IIb Acute pelvic infections and Complicated intra-abdominal infections trials in which patients 3 months to 12 years of age received INVANZ 15 mg/kg IV twice daily up to a maximum of 1 g and patients 13 to 17 years of age received INVANZ 1 g IV daily or ticarcillin/clavulanate 50 mg/kg for patients <60 kg or ticarcillin/clavulanate 3.0 g for patients >60 kg, 4 or 6 times a day.
Local: Infusion Site Erythema 3.9 3.0 8.3 Infusion Site Pain 7.0 4.0 20.8 Systemic: Abdominal Pain 4.7 3.0 4.2 Constipation 2.3 0.0 0.0 Diarrhea 11.7 17.0 4.2 Loose Stools 2.1 0.0 0.0 Vomiting 10.2 11.0 8.3 Pyrexia 4.9 6.0 8.3 Upper Respiratory Tract Infection 2.3 3.0 0.0 Headache 4.4 4.0 0.0 Cough 4.4 3.0 0.0 Diaper Dermatitis 4.7 4.0 0.0 Rash 2.9 2.0 8.3 Additional adverse experiences that were reported with INVANZ with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders: nausea
General Disorders and Administration Site Condition: hypothermia, chest pain, upper abdominal pain; infusion site pruritus, induration, phlebitis, swelling, and warmth
Infections and Infestations: candidiasis, oral candidiasis, viral pharyngitis, herpes simplex, ear infection, abdominal abscess
Metabolism and Nutrition Disorders: decreased appetite
Musculoskeletal and Connective Tissue Disorders: arthralgia
Nervous System Disorders: dizziness, somnolence
Psychiatric Disorders: insomnia
Reproductive System and Breast Disorders: genital rash
Respiratory, Thoracic and Mediastinal Disorders: wheezing, nasopharyngitis, pleural effusion, rhinitis, rhinorrhea
Skin and Subcutaneous Tissue Disorders: dermatitis, pruritus, rash erythematous, skin lesion
Vascular Disorders: phlebitis.
6.2 Post-Marketing Experience
The following additional adverse reactions have been identified during the post-approval use of INVANZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: teeth staining
Immune System Disorders: anaphylaxis including anaphylactoid reactions
Musculoskeletal and Connective Tissue Disorders: muscular weakness
Nervous System Disorders: coordination abnormal, depressed level of consciousness, dyskinesia, gait disturbance, myoclonus, tremor, encephalopathy (recovery was prolonged in patients with renal impairment)
Psychiatric Disorders: altered mental status (including aggression, delirium), hallucinations
Skin and Subcutaneous Tissue Disorders: Acute Generalized Exanthematous Pustulosis (AGEP), Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS syndrome), hypersensitivity vasculitis
Close6.3 Adverse Laboratory Changes in Clinical Trials
Adults Receiving INVANZ as Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ in clinical trials are presented in Table 6. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were ALT increased (6.0%), AST increased (5.2%), serum alkaline phosphatase increased (3.4%), and platelet count increased (2.8%). INVANZ was discontinued due to laboratory adverse experiences in 0.3% of patients.
Table 6 Incidence* (%) of Laboratory Adverse Experiences Reported During Study Therapy Plus 14-Day Follow-Up in ≥2.0% of Adult Patients Treated With INVANZ in Clinical Trials INVANZ†
1 g dailyPiperacillin/
Tazobactam†
3.375 g q6hINVANZ‡
1 g dailyCeftriaxone‡
1 or 2 g dailyAdverse laboratory experiences (n§=766) (n§=755) (n§=1122) (n§=920) - *
- Number of patients with laboratory adverse experiences/Number of patients with the laboratory test
- †
- Includes Phase IIb/III Complicated intra-abdominal infections, Complicated skin and skin structure infections and Acute pelvic infections trials
- ‡
- Includes Phase IIb/III Community acquired pneumonia and Complicated urinary tract infections, and Phase IIa trials
- §
- Number of patients with one or more laboratory tests
ALT increased 8.8 7.3 8.3 6.9 AST increased 8.4 8.3 7.1 6.5 Serum alkaline phosphatase increased 6.6 7.2 4.3 2.8 Eosinophils increased 1.1 1.1 2.1 1.8 Hematocrit decreased 3.0 2.9 3.4 2.4 Hemoglobin decreased 4.9 4.7 4.5 3.5 Platelet count increased 6.5 6.3 4.3 3.5 Urine RBCs increased 2.5 2.9 1.1 1.0 Urine WBCs increased 2.5 3.2 1.6 1.1 Additional laboratory adverse experiences that were reported during therapy in >0.1% of patients treated with INVANZ in clinical trials include: increases in serum creatinine, serum glucose, BUN, total, direct and indirect serum bilirubin, serum sodium and potassium, PT and PTT; decreases in serum potassium, serum albumin, WBC, platelet count, and segmented neutrophils.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the laboratory adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post-surgery, the overall laboratory adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ in clinical trials are presented in Table 7. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were neutrophil count decreased (3.0%), ALT increased (2.2%), and AST increased (2.1%).
Table 7 Incidence* (%) of Specific Laboratory Adverse Experiences Reported During Study Therapy Plus 14-Day Follow-Up in ≥2.0% of Pediatric Patients Treated With INVANZ in Clinical Trials INVANZ Ceftriaxone Ticarcillin/
ClavulanateAdverse laboratory experiences (n†=379) (n†=97) (n†=24) ALT Increased 3.8 1.1 4.3 AST Increased 3.8 1.1 4.3 Neutrophil Count Decreased 5.8 3.1 0.0 Additional laboratory adverse experiences that were reported during therapy in >0.5% of patients treated with INVANZ in clinical trials include: alkaline phosphatase increased, eosinophil count increased, platelet count increased, white blood cell count decreased and urine protein present.
-
7 DRUG INTERACTIONS7.1 Probenecid - Probenecid interferes with the active tubular secretion of ertapenem, resulting in increased plasma concentrations of ertapenem [see Clinical Pharmacology (12.3)] ...
7.1 Probenecid
Probenecid interferes with the active tubular secretion of ertapenem, resulting in increased plasma concentrations of ertapenem [see Clinical Pharmacology (12.3)]. Co-administration of probenecid with ertapenem is not recommended.
Close7.2 Valproic Acid
Case reports in the literature have shown that co-administration of carbapenems, including ertapenem, to patients receiving valproic acid or divalproex sodium results in a reduction of valproic acid concentrations. The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures. Although the mechanism of this interaction is unknown, data from in vitro and animal studies suggest that carbapenems may inhibit the hydrolysis of valproic acid's glucuronide metabolite (VPA-g) back to valproic acid, thus decreasing the serum concentrations of valproic acid [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from a small number of post-marketing cases with INVANZ use in pregnancy are insufficient to inform any drug-associated risks for major birth ...
8.1 Pregnancy
Risk Summary
Available data from a small number of post-marketing cases with INVANZ use in pregnancy are insufficient to inform any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies after intravenous administration of ertapenem during the period of organogenesis, there was no evidence of developmental malformations in rats at systemic exposures (AUC) up to approximately 1.2 times the human exposure at the maximum recommended human dose (MRHD) and in mice at doses up to approximately 3 times the MRHD based on body surface area comparison. In pregnant rats administered ertapenem during organogenesis through lactation, fetal toxicity, developmental delays, and impaired reproduction did not occur in first generation offspring at systemic exposures (AUC) approximately 1.2 times the human exposure at the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In pregnant rats, intravenous administration of ertapenem dosages of up to 700 mg/kg/day (approximately 1.2 times the MRHD based on AUC) during the period of organogenesis (gestation days [GD] 6-20) revealed no maternal or embryofetal effects.
Pregnant mice intravenously administered ertapenem dosages of up to 700 mg/kg/day (approximately 3 times the MRHD based on body surface area comparison) during the period of organogenesis (GD 6-15) showed slight decreases in average fetal weight and an associated decrease in the average number of ossified sacrocaudal vertebrae. There were no maternal effects at any dosage.
In a pre-postnatal study in rats, ertapenem administered to pregnant rats at dosages up to 700 mg/kg/day (approximately 1.2 times the MRHD based on AUC) during organogenesis through lactation, (GD 6 until Lactation Day (LD) 20) did not result in fetal toxicity, developmental delays, or impaired reproduction in first generation offspring, and fetal deaths and malformations were not increased in second generation offspring.
8.2 Lactation
Risk Summary
Ertapenem is present in human milk (see Data). There are no data on the effects on the breastfed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for INVANZ and any potential adverse effects on the breastfed infant from INVANZ or from the underlying maternal condition.
Data
The concentration of ertapenem in breast milk from 5 lactating women with pelvic infections (5 to 14 days postpartum) measured at random time points daily for 5 consecutive days following the last 1 g dose of intravenous therapy (3 to 10 days of therapy) showed low levels. The concentration of ertapenem in breast milk within 24 hours of the last dose of therapy in all 5 women ranged from (<0.13 (lower limit of quantitation) to 0.38 mcg/mL), although peak concentrations were not assessed. By day 5 after discontinuation of therapy, the level of ertapenem was undetectable in the breast milk of 4 women and below the lower limit of quantitation (<0.13 mcg/mL) in 1 woman. The concentration of ertapenem in transitional milk observed in this study may not reflect the concentration of ertapenem in mature milk.
8.4 Pediatric Use
Safety and effectiveness of INVANZ in pediatric patients 3 months to 17 years of age are supported by evidence from adequate and well-controlled trials in adults, pharmacokinetic data in pediatric patients, and additional data from comparator-controlled trials in pediatric patients 3 months to 17 years of age [see Indications and Usage (1.1), (1.2), (1.3), (1.4) and (1.5) and Clinical Studies (14.2)].
INVANZ is not recommended in infants under 3 months of age as no data are available.
INVANZ is not recommended in the treatment of meningitis in the pediatric population due to lack of sufficient CSF penetration.
8.5 Geriatric Use
Of the 1,835 patients in Phase 2b/3 trials treated with INVANZ, approximately 26 percent were 65 and over, while approximately 12 percent were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.2)].
8.6 Patients with Renal Impairment
Dosage adjustment is necessary in patients with creatinine clearance 30 mL/min or less [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Close8.7 Patients with Hepatic Impairment
The pharmacokinetics of ertapenem in patients with hepatic impairment have not been established. Of the total number of patients in clinical trials, 37 patients receiving ertapenem 1 g daily and 36 patients receiving comparator drugs were considered to have Child-Pugh Class A, B, or C liver impairment. The incidence of adverse experiences in patients with hepatic impairment was similar between the ertapenem group and the comparator groups.
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage with INVANZ. Intentional overdosing of INVANZ is unlikely. Intravenous administration of INVANZ at a dose of 2 g over 30 min or ...
No specific information is available on the treatment of overdosage with INVANZ. Intentional overdosing of INVANZ is unlikely. Intravenous administration of INVANZ at a dose of 2 g over 30 min or 3 g over 1-2h in healthy adult volunteers resulted in an increased incidence of nausea. In clinical trials in adults, inadvertent administration of three 1 g doses of INVANZ in a 24 hour period resulted in diarrhea and transient dizziness in one patient. In pediatric clinical trials, a single intravenous dose of 40 mg/kg up to a maximum of 2 g did not result in toxicity.
In the event of an overdose, INVANZ should be discontinued and general supportive treatment given until renal elimination takes place.
INVANZ can be removed by hemodialysis; the plasma clearance of the total fraction of ertapenem was increased 30% in subjects with end-stage renal disease when hemodialysis (4 hour session) was performed immediately following administration. However, no information is available on the use of hemodialysis to treat overdosage.
Close -
11 DESCRIPTIONINVANZ (Ertapenem for Injection) is a sterile, synthetic, parenteral, 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics. Chemically, INVANZ is described as ...
INVANZ (Ertapenem for Injection) is a sterile, synthetic, parenteral, 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics.
Chemically, INVANZ is described as [4R-[3(3S*,5S*),4α,5β,6β(R*)]]-3-[[5-[[(3-carboxyphenyl)amino]carbonyl]-3-pyrrolidinyl]thio]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt. Its molecular weight is 497.50. The empirical formula is C22H24N3O7SNa, and its structural formula is:
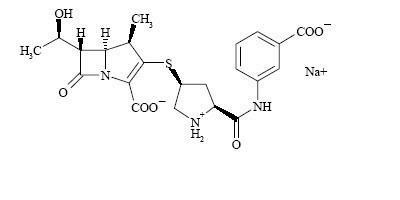
Ertapenem sodium is a white to off-white hygroscopic, weakly crystalline powder. It is soluble in water and 0.9% sodium chloride solution, practically insoluble in ethanol, and insoluble in isopropyl acetate and tetrahydrofuran.
INVANZ is supplied as sterile lyophilized powder for intravenous infusion after reconstitution with appropriate diluent [see Dosage and Administration (2.7)] and transfer to 50 mL 0.9% Sodium Chloride Injection or for intramuscular injection following reconstitution with 1% lidocaine hydrochloride. Each single-dose vial contains 1 gram ertapenem equivalent to 1.046 grams ertapenem sodium. The sodium content is approximately 137 mg (approximately 6.0 mEq).
Each vial of INVANZ contains the following inactive ingredients: 175 mg sodium bicarbonate and sodium hydroxide to adjust pH to 7.5.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ertapenem sodium is a carbapenem antibiotic [see Clinical Pharmacology (12.4)]. 12.3 Pharmacokinetics - Average plasma concentrations (mcg/mL) of ertapenem following ...
12.1 Mechanism of Action
Ertapenem sodium is a carbapenem antibiotic [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Average plasma concentrations (mcg/mL) of ertapenem following a single 30-minute infusion of a 1 g intravenous (IV) dose and administration of a single 1 g intramuscular (IM) dose in healthy young adults are presented in Table 8.
Table 8 Plasma Concentrations of Ertapenem in Adults After Single Dose Administration Average Plasma Concentrations (mcg/mL) Dose/Route 0.5 hr 1 hr 2 hr 4 hr 6 hr 8 hr 12 hr 18 hr 24 hr - *
- Infused at a constant rate over 30 minutes
1 g IV* 155 115 83 48 31 20 9 3 1 1 g IM 33 53 67 57 40 27 13 4 2 The area under the plasma concentration-time curve (AUC) of ertapenem in adults increased less-than dose-proportional based on total ertapenem concentrations over the 0.5 to 2 g dose range, whereas the AUC increased greater-than dose-proportional based on unbound ertapenem concentrations. Ertapenem exhibits non-linear pharmacokinetics due to concentration-dependent plasma protein binding at the proposed therapeutic dose [see Clinical Pharmacology (12.3)]. There is no accumulation of ertapenem following multiple IV or IM 1 g daily doses in healthy adults.
Average plasma concentrations (mcg/mL) of ertapenem in pediatric patients are presented in Table 9.
Table 9 Plasma Concentrations of Ertapenem in Pediatric Patients After Single IV* Dose Administration Age Group Dose Average Plasma Concentrations (mcg/mL) 0.5 hr 1 hr 2 hr 4 hr 6 hr 8 hr 12 hr 24 hr 3 to 23 months
15 mg/kg†
103.8
57.3
43.6
23.7
13.5
8.2
2.5
-20 mg/kg† 126.8 87.6 58.7 28.4 - 12.0 3.4 0.4 40 mg/kg‡ 199.1 144.1 95.7 58.0 - 20.2 7.7 0.6 2 to 12 years
15 mg/kg†
113.2
63.9
42.1
21.9
12.8
7.6
3.0
-20 mg/kg† 147.6 97.6 63.2 34.5 - 12.3 4.9 0.5 40 mg/kg‡ 241.7 152.7 96.3 55.6 - 18.8 7.2 0.6 13 to 17 years
20 mg/kg†
170.4
98.3
67.8
40.4
-
16.0
7.0
1.11 g§ 155.9 110.9 74.8 - 24.0 - 6.2 - 40 mg/kg‡ 255.0 188.7 127.9 76.2 - 31.0 15.3 2.1 Absorption
Ertapenem, reconstituted with 1% lidocaine HCl injection, USP (in saline without epinephrine), is almost completely absorbed following intramuscular (IM) administration at the recommended dose of 1 g. The mean bioavailability is approximately 90%. Following 1 g daily IM administration, mean peak plasma concentrations (Cmax) are achieved in approximately 2.3 hours (Tmax).
Distribution
Ertapenem is highly bound to human plasma proteins, primarily albumin. In healthy young adults, the protein binding of ertapenem decreases as plasma concentrations increase, from approximately 95% bound at an approximate plasma concentration of <100 micrograms (mcg)/mL to approximately 85% bound at an approximate plasma concentration of 300 mcg/mL.
The apparent volume of distribution at steady state (Vss) of ertapenem in adults is approximately 0.12 liter/kg, approximately 0.2 liter/kg in pediatric patients 3 months to 12 years of age and approximately 0.16 liter/kg in pediatric patients 13 to 17 years of age.
The concentrations of ertapenem achieved in suction-induced skin blister fluid at each sampling point on the third day of 1 g once daily IV doses are presented in Table 10. The ratio of AUC0-24 in skin blister fluid/AUC0-24 in plasma is 0.61.
Table 10 Concentrations (mcg/mL) of Ertapenem in Adult Skin Blister Fluid at each Sampling Point on the Third Day of 1-g Once Daily IV Doses 0.5 hr 1 hr 2 hr 4 hr 8 hr 12 hr 24 hr 7 12 17 24 24 21 8 Metabolism
In healthy young adults, after infusion of 1 g IV radiolabeled ertapenem, the plasma radioactivity consists predominantly (94%) of ertapenem. The major metabolite of ertapenem is the inactive ring-opened derivative formed by hydrolysis of the beta-lactam ring.
Elimination
Ertapenem is eliminated primarily by the kidneys. The mean plasma half-life in healthy young adults is approximately 4 hours and the plasma clearance is approximately 1.8 L/hour. The mean plasma half-life in pediatric patients 13 to 17 years of age is approximately 4 hours and approximately 2.5 hours in pediatric patients 3 months to 12 years of age.
Following the administration of 1 g IV radiolabeled ertapenem to healthy young adults, approximately 80% is recovered in urine and 10% in feces. Of the 80% recovered in urine, approximately 38% is excreted as unchanged drug and approximately 37% as the ring-opened metabolite.
In healthy young adults given a 1 g IV dose, the mean percentage of the administered dose excreted in urine was 17.4% during 0-2 hours postdose, 5.4% during 4-6 hours postdose, and 2.4% during 12-24 hours postdose.
Special Populations
Renal Impairment
Total and unbound fractions of ertapenem pharmacokinetics were investigated in 26 adult subjects (31 to 80 years of age) with varying degrees of renal impairment. Following a single 1 g IV dose of ertapenem, the unbound AUC increased 1.5-fold and 2.3-fold in subjects with mild renal impairment (CLCR 60-90 mL/min/1.73 m2) and moderate renal impairment (CLCR 31-59 mL/min/1.73 m2), respectively, compared with healthy young subjects (25 to 45 years of age). No dosage adjustment is necessary in patients with CLCR ≥31 mL/min/1.73 m2. The unbound AUC increased 4.4-fold and 7.6-fold in subjects with advanced renal impairment (CLCR 5-30 mL/min/1.73 m2) and end-stage renal disease (CLCR <10 mL/min/1.73 m2), respectively, compared with healthy young subjects. The effects of renal impairment on AUC of total drug were of smaller magnitude. The recommended dose of ertapenem in adult patients with CLCR ≤30 mL/min/1.73 m2 is 0.5 grams every 24 hours. Following a single 1 g IV dose given immediately prior to a 4 hour hemodialysis session in 5 adult patients with end-stage renal disease, approximately 30% of the dose was recovered in the dialysate. Dose adjustments are recommended for patients with severe renal impairment and end-stage renal disease [see Dosage and Administration (2.4)]. There are no data in pediatric patients with renal impairment.
Hepatic Impairment
The pharmacokinetics of ertapenem in patients with hepatic impairment have not been established. However, ertapenem does not appear to undergo hepatic metabolism based on in vitro studies and approximately 10% of an administered dose is recovered in the feces [see Clinical Pharmacology (12.3) and Dosage and Administration (2.6)].
Gender
The effect of gender on the pharmacokinetics of ertapenem was evaluated in healthy male (n=8) and healthy female (n=8) subjects. The differences observed could be attributed to body size when body weight was taken into consideration. No dose adjustment is recommended based on gender.
Geriatric Patients
The impact of age on the pharmacokinetics of ertapenem was evaluated in healthy male (n=7) and healthy female (n=7) subjects ≥65 years of age. The total and unbound AUC increased 37% and 67%, respectively, in elderly adults relative to young adults. These changes were attributed to age-related changes in creatinine clearance. No dosage adjustment is necessary for elderly patients with normal (for their age) renal function.
Pediatric Patients
Plasma concentrations of ertapenem are comparable in pediatric patients 13 to 17 years of age and adults following a 1 g once daily IV dose.
Following the 20 mg/kg dose (up to a maximum dose of 1 g), the pharmacokinetic parameter values in patients 13 to 17 years of age (N=6) were generally comparable to those in healthy young adults.
Plasma concentrations at the midpoint of the dosing interval following a single 15 mg/kg IV dose of ertapenem in patients 3 months to 12 years of age are comparable to plasma concentrations at the midpoint of the dosing interval following a 1 g once daily IV dose in adults [see Clinical Pharmacology (12.3)]. The plasma clearance (mL/min/kg) of ertapenem in patients 3 months to 12 years of age is approximately 2-fold higher as compared to that in adults. At the 15 mg/kg dose, the AUC value (doubled to model a twice daily dosing regimen, i.e., 30 mg/kg/day exposure) in patients 3 months to 12 years of age was comparable to the AUC value in young healthy adults receiving a 1 g IV dose of ertapenem.
Drug Interactions
When ertapenem is co-administered with probenecid (500 mg p.o. every 6 hours), probenecid competes for active tubular secretion and reduces the renal clearance of ertapenem. Based on total ertapenem concentrations, probenecid increased the AUC of ertapenem by 25%, and reduced the plasma and renal clearance of ertapenem by 20% and 35%, respectively. The half-life of ertapenem was increased from 4.0 to 4.8 hours.
In vitro studies in human liver microsomes indicate that ertapenem does not inhibit metabolism mediated by any of the following cytochrome p450 (CYP) isoforms: 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4.
In vitro studies indicate that ertapenem does not inhibit P-glycoprotein-mediated transport of digoxin or vinblastine and that ertapenem is not a substrate for P-glycoprotein-mediated transport.
Close12.4 Microbiology
Mechanism of Action
Ertapenem has in vitro activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. The bactericidal activity of ertapenem results from the inhibition of cell wall synthesis and is mediated through ertapenem binding to penicillin binding proteins (PBPs). In Escherichia coli, it has strong affinity toward PBPs 1a, 1b, 2, 3, 4 and 5 with preference for PBPs 2 and 3.
Resistance
Ertapenem is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases. Ertapenem is hydrolyzed by metallo-beta-lactamases.
Antimicrobial Activity
Ertapenem has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Gram-positive bacteria:
Staphylococcus aureus (methicillin susceptible isolates only)
Streptococcus agalactiae
Streptococcus pneumoniae (penicillin susceptible isolates only)
Streptococcus pyogenesGram-negative bacteria:
Escherichia coli
Haemophilus influenzae (beta-lactamase negative isolates only)
Klebsiella pneumoniae
Moraxella catarrhalis
Proteus mirabilisAnaerobic bacteria:
Bacteroides fragilis
Bacteroides distasonis
Bacteroides ovatus
Bacteroides thetaiotaomicron
Bacteroides uniformis
Clostridium clostridioforme
Eubacterium lentum
Peptostreptococcus species
Porphyromonas asaccharolytica
Prevotella biviaThe following in vitro data are available, but their clinical significance is unknown. At least 90% of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for ertapenem. However, the efficacy of ertapenem in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials:
Gram-positive bacteria:
Staphylococcus epidermidis (methicillin susceptible isolates only)
Streptococcus pneumoniae (penicillin-intermediate isolates)Gram-negative bacteria:
Citrobacter freundii
Citrobacter koseri
Enterobacter aerogenes
Enterobacter cloacae
Haemophilus influenzae (beta-lactamase positive isolates only)
Haemophilus parainfluenzae
Klebsiella oxytoca (excluding ESBL producing isolates)
Morganella morganii
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Serratia marcescensAnaerobic bacteria:
Bacteroides vulgatus
Clostridium perfringens
Fusobacterium spp. -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis - No long-term studies in animals have been performed to evaluate the carcinogenic potential of ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
No long-term studies in animals have been performed to evaluate the carcinogenic potential of ertapenem.
Ertapenem was not genotoxic in in vitro or in vivo assays, including: an alkaline elution/rat hepatocyte assay, a chromosomal aberration assay in Chinese hamster ovary cells, a TK6 human lymphoblastoid cell mutagenesis assay and a mouse micronucleus assay.
Close13.2 Animal Toxicology and/or Pharmacology
In repeat-dose studies in rats, treatment-related neutropenia occurred at every dose-level tested, including the lowest dose of 2 mg/kg (approximately 2% of the human dose on a body surface area basis).
Studies in rabbits and Rhesus monkeys were inconclusive with regard to the effect on neutrophil counts.
-
14 CLINICAL STUDIES14.1 Adults - Complicated Intra-Abdominal Infections - Ertapenem was evaluated in adults for the treatment of complicated intra-abdominal infections in a randomized, double-blind, non-inferiority ...
14.1 Adults
Complicated Intra-Abdominal Infections
Ertapenem was evaluated in adults for the treatment of complicated intra-abdominal infections in a randomized, double-blind, non-inferiority clinical trial. This trial compared ertapenem (1 g intravenously once a day) with piperacillin/tazobactam (3.375 g intravenously every 6 hours) for 5 to 14 days and enrolled 665 patients with localized complicated appendicitis, and any other complicated intra-abdominal infection including colonic, small intestinal, and biliary infections and generalized peritonitis. The combined clinical and microbiologic success rates in the microbiologically evaluable population at 4 to 6 weeks posttherapy (test-of-cure) were 83.6% (163/195) for ertapenem and 80.4% (152/189) for piperacillin/tazobactam.
Complicated Skin and Skin Structure Infections
Ertapenem was evaluated in adults for the treatment of complicated skin and skin structure infections in a randomized, double-blind, non-inferiority clinical trial. This trial compared ertapenem (1 g intravenously once a day) with piperacillin/tazobactam (3.375 g intravenously every 6 hours) for 7 to 14 days and enrolled 540 patients including patients with deep soft tissue abscess, posttraumatic wound infection and cellulitis with purulent drainage. The clinical success rates at 10 to 21 days posttherapy (test-of-cure) were 83.9% (141/168) for ertapenem and 85.3% (145/170) for piperacillin/tazobactam.
Diabetic Foot Infections
Ertapenem was evaluated in adults for the treatment of diabetic foot infections without concomitant osteomyelitis in a multicenter, randomized, double-blind, non-inferiority clinical trial. This trial compared ertapenem (1 g intravenously once a day) with piperacillin/tazobactam (3.375 g intravenously every 6 hours). Test-of-cure was defined as clinical response between treatment groups in the clinically evaluable population at the 10-day posttherapy follow-up visit. The trial included 295 patients randomized to ertapenem and 291 patients to piperacillin/tazobactam. Both regimens allowed the option to switch to oral amoxicillin/clavulanate for a total of 5 to 28 days of treatment (parenteral and oral). All patients were eligible to receive appropriate adjunctive treatment methods, such as debridement, as is typically required in the treatment of diabetic foot infections, and most patients received these treatments. Patients with suspected osteomyelitis could be enrolled if all the infected bone was removed within 2 days of initiation of study therapy, and preferably within the prestudy period. Investigators had the option to add open-label vancomycin if enterococci or methicillin-resistant Staphylococcus aureus (MRSA) were among the pathogens isolated or if patients had a history of MRSA infection and additional therapy was indicated in the opinion of the investigator. Two hundred and four (204) patients randomized to ertapenem and 202 patients randomized to piperacillin/tazobactam were clinically evaluable. The clinical success rates at 10 days posttherapy were 75.0% (153/204) for ertapenem and 70.8% (143/202) for piperacillin/tazobactam.
Community Acquired Pneumonia
Ertapenem was evaluated in adults for the treatment of community acquired pneumonia in two randomized, double-blind, non-inferiority clinical trials. Both trials compared ertapenem (1 g parenterally once a day) with ceftriaxone (1 g parenterally once a day) and enrolled a total of 866 patients. Both regimens allowed the option to switch to oral amoxicillin/clavulanate for a total of 10 to 14 days of treatment (parenteral and oral). In the first trial the primary efficacy parameter was the clinical success rate in the clinically evaluable population and success rates were 92.3% (168/182) for ertapenem and 91.0% (183/201) for ceftriaxone at 7 to 14 days posttherapy (test-of-cure). In the second trial the primary efficacy parameter was the clinical success rate in the microbiologically evaluable population and success rates were 91% (91/100) for ertapenem and 91.8% (45/49) for ceftriaxone at 7 to 14 days posttherapy (test-of-cure).
Complicated Urinary Tract Infections Including Pyelonephritis
Ertapenem was evaluated in adults for the treatment of complicated urinary tract infections including pyelonephritis in two randomized, double-blind, non-inferiority clinical trials. Both trials compared ertapenem (1 g parenterally once a day) with ceftriaxone (1 g parenterally once a day) and enrolled a total of 850 patients. Both regimens allowed the option to switch to oral ciprofloxacin (500 mg twice daily) for a total of 10 to 14 days of treatment (parenteral and oral). The microbiological success rates (combined trials) at 5 to 9 days posttherapy (test-of-cure) were 89.5% (229/256) for ertapenem and 91.1% (204/224) for ceftriaxone.
Acute Pelvic Infections Including Endomyometritis, Septic Abortion and Post-Surgical Gynecological Infections
Ertapenem was evaluated in adults for the treatment of acute pelvic infections in a randomized, double-blind, non-inferiority clinical trial. This trial compared ertapenem (1 g intravenously once a day) with piperacillin/tazobactam (3.375 g intravenously every 6 hours) for 3 to 10 days and enrolled 412 patients including 350 patients with obstetric/postpartum infections and 45 patients with septic abortion. The clinical success rates in the clinically evaluable population at 2 to 4 weeks posttherapy (test-of-cure) were 93.9% (153/163) for ertapenem and 91.5% (140/153) for piperacillin/tazobactam.
Prophylaxis of Surgical Site Infections Following Elective Colorectal Surgery
Ertapenem was evaluated in adults for prophylaxis of surgical site infection following elective colorectal surgery in a multicenter, randomized, double-blind, non-inferiority clinical trial. This trial compared a single intravenous dose of ertapenem (1 g) versus cefotetan (2 g) administered over 30 minutes, 1 hour before elective colorectal surgery. Test-of-prophylaxis was defined as no evidence of surgical site infection, post-operative anastomotic leak, or unexplained antibiotic use in the clinically evaluable population up to and including at the 4-week posttreatment follow-up visit. The trial included 500 patients randomized to ertapenem and 502 patients randomized to cefotetan. The modified intent-to-treat (MITT) population consisted of 451 ertapenem patients and 450 cefotetan patients and included all patients who were randomized, treated, and underwent elective colorectal surgery with adequate bowel preparation. The clinically evaluable population was a subset of the MITT population and consisted of patients who received a complete dose of study therapy no more than two hours prior to surgical incision and no more than six hours before surgical closure. Clinically evaluable patients had sufficient information to determine outcome at the 4-week follow-up assessment and had no confounding factors that interfered with the assessment of that outcome. Examples of confounding factors included prior or concomitant antibiotic violations, the need for a second surgical procedure during the study period, and identification of a distant site infection with concomitant antibiotic administration and no evidence of subsequent wound infection. Three-hundred forty-six (346) patients randomized to ertapenem and 339 patients randomized to cefotetan were clinically evaluable. The prophylactic success rates at 4 weeks posttreatment in the clinically evaluable population were 70.5% (244/346) for ertapenem and 57.2% (194/339) for cefotetan (difference 13.3%, [95% C.I.: 6.1, 20.4], p<0.001). Prophylaxis failure due to surgical site infections occurred in 18.2% (63/346) ertapenem patients and 31.0% (105/339) cefotetan patients. Post-operative anastomotic leak occurred in 2.9% (10/346) ertapenem patients and 4.1% (14/339) cefotetan patients. Unexplained antibiotic use occurred in 8.4% (29/346) ertapenem patients and 7.7% (26/339) cefotetan patients. Though patient numbers were small in some subgroups, in general, clinical response rates by age, gender, and race were consistent with the results found in the clinically evaluable population. In the MITT analysis, the prophylactic success rates at 4 weeks posttreatment were 58.3% (263/451) for ertapenem and 48.9% (220/450) for cefotetan (difference 9.4%, [95% C.I.: 2.9, 15.9], p=0.002). A statistically significant difference favoring ertapenem over cefotetan with respect to the primary endpoint has been observed at a significance level of 5% in this trial. A second adequate and well-controlled trial to confirm these findings has not been conducted; therefore, the clinical superiority of ertapenem over cefotetan has not been demonstrated.
Close14.2 Pediatric Patients
Ertapenem was evaluated in pediatric patients 3 months to 17 years of age in two randomized, multicenter clinical trials.
The first trial enrolled 404 patients and compared ertapenem (15 mg/kg intravenous (IV) every 12 hours in patients 3 months to 12 years of age, and 1 g IV once a day in patients 13 to 17 years of age) to ceftriaxone (50 mg/kg/day IV in two divided doses in patients 3 months to 12 years of age and 50 mg/kg/day IV as a single daily dose in patients 13 to 17 years of age) for the treatment of complicated urinary tract infection (UTI), skin and soft tissue infection (SSTI), or community-acquired pneumonia (CAP). Both regimens allowed the option to switch to oral amoxicillin/clavulanate for a total of up to 14 days of treatment (parenteral and oral). The microbiological success rates in the evaluable per protocol (EPP) analysis in patients treated for UTI were 87.0% (40/46) for ertapenem and 90.0% (18/20) for ceftriaxone. The clinical success rates in the EPP analysis in patients treated for SSTI were 95.5% (64/67) for ertapenem and 100% (26/26) for ceftriaxone, and in patients treated for CAP were 96.1% (74/77) for ertapenem and 96.4% (27/28) for ceftriaxone.
The second trial enrolled 112 patients and compared ertapenem (15 mg/kg IV every 12 hours in patients 3 months to 12 years of age, and 1 g IV once a day in patients 13 to 17 years of age) to ticarcillin/clavulanate (50 mg/kg for patients <60 kg or 3.0 g for patients >60 kg, 4 or 6 times a day) up to 14 days for the treatment of complicated intra-abdominal infections (IAI) and acute pelvic infections (API). In patients treated for IAI (primarily patients with perforated or complicated appendicitis), the clinical success rates were 83.7% (36/43) for ertapenem and 63.6% (7/11) for ticarcillin/clavulanate in the EPP analysis. In patients treated for API (post-operative or spontaneous obstetrical endomyometritis, or septic abortion), the clinical success rates were 100% (23/23) for ertapenem and 100% (4/4) for ticarcillin/clavulanate in the EPP analysis.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - INVANZ is supplied as a sterile lyophilized powder in single-dose vials containing ertapenem for intravenous infusion or for intramuscular injection as follows: 1 g ertapenem ...
16.1 How Supplied
INVANZ is supplied as a sterile lyophilized powder in single-dose vials containing ertapenem for intravenous infusion or for intramuscular injection as follows:
1 g ertapenem equivalent
NDC 0006-3843-71 in packages of 10 vials.
Close16.2 Storage and Handling
Before reconstitution
Do not store lyophilized powder above 25°C (77°F).
Reconstituted and infusion solutions
The reconstituted solution, immediately diluted in 0.9% Sodium Chloride Injection [see Dosage and Administration (2.7)], may be stored at room temperature (25°C) and used within 6 hours or stored for 24 hours under refrigeration (5°C) and used within 4 hours after removal from refrigeration. Solutions of INVANZ should not be frozen.
-
17 PATIENT COUNSELING INFORMATION17.1 Instructions for Patients - Patients should be advised that allergic reactions, including serious allergic reactions could occur and that serious reactions may require immediate treatment ...Close
17.1 Instructions for Patients
Patients should be advised that allergic reactions, including serious allergic reactions could occur and that serious reactions may require immediate treatment. Advise patients to report any previous hypersensitivity reactions to INVANZ, other beta-lactams or other allergens.
Patients should be counseled to inform their physician if they are taking valproic acid or divalproex sodium. Valproic acid concentrations in the blood may drop below the therapeutic range upon co-administration with INVANZ. If treatment with INVANZ is necessary and continued, alternative or supplemental anti-convulsant medication to prevent and/or treat seizures may be needed.
Patients should be counseled that antibacterial drugs including INVANZ should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When INVANZ is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by INVANZ or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
-
SPL UNCLASSIFIED SECTIONManuf. for: Merck Sharp & Dohme LLC - Rahway, NJ 07065, USA - For patent information: www.msd.com/research/patent - Copyright © 2001-2022 Merck & Co., Inc., Rahway, NJ, USA, and its ...
Manuf. for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2001-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk0826-i-2211r029
Close -
PRINCIPAL DISPLAY PANEL - 10 Single-dose vials LabelNDC 0006-3843-71 - 10 Single-dose vials - 1 g - FOR INSTRUCTIONS ON RECONSTITUTION - AND DILUTION and USUAL DOSAGE: Discard vial with unused portion. See accompanying circular. Each vial contains 1 ...
NDC 0006-3843-71
10 Single-dose vials
1 gFOR INSTRUCTIONS ON RECONSTITUTION
AND DILUTION and USUAL DOSAGE:
Discard vial with unused portion.
See accompanying circular.Each vial contains 1 gram ertapenem,
equivalent to 1.046 grams ertapenem sodium.INVANZ®
(ertapenem sodium) IV/IMFOR INTRAVENOUS OR
INTRAMUSCULAR USERx only
Inactive ingredients: 175 mg sodium
bicarbonate and sodium hydroxide to
adjust pH to 7.5.Solutions range from colorless to pale
yellow. Variations of color within this
range do not affect the potency of the
product.Do not store lyophilized powder above
25°C (77°F).MERCK
Manuf. for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USABy: Fareva Mirabel
Route de Marsat, Riom, 63200, France
Formulated in France.Close
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 1 Single-dose vial Label - NDC 0006-3843-71 - Do not store lyophilized powder - above 25°C (77°F). FOR INSTRUCTIONS ON - RECONSTITUTION AND DILUTION - and USUAL DOSAGE: See ...
PRINCIPAL DISPLAY PANEL - 1 Single-dose vial Label
NDC 0006-3843-71
Do not store lyophilized powder
above 25°C (77°F).FOR INSTRUCTIONS ON
RECONSTITUTION AND DILUTION
and USUAL DOSAGE:See accompanying circular.
Each vial contains 1 gram
ertapenem, equivalent to
1.046 grams ertapenem sodium.Formulated in France.
1 Single-dose vial. Discard unused portion.
1 gRx only
INVANZ®
(ertapenem sodium) IV/IMInactive ingredients: 175 mg sodium bicarbonate and sodium hydroxide to
adjust pH to 7.5. Solutions range from colorless to pale yellow. Variations
of color within this range do not affect the potency of the product.FOR INTRAVENOUS OR INTRAMUSCULAR USE
Manuf. for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USABy: Fareva Mirabel
Route de Marsat, Riom, 63200, France7008133403
180522-1Close
-
INGREDIENTS AND APPEARANCEProduct Information
INVANZ ertapenem sodium injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-3843 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERTAPENEM SODIUM (UNII: 2T90KE67L0) (ertapenem - UNII:G32F6EID2H) ertapenem 1 g Inactive Ingredients Ingredient Name Strength sodium bicarbonate (UNII: 8MDF5V39QO) 175 mg sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-3843-71 10 in 1 PACKAGE 11/21/2001 1 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021337 11/21/2001 INVANZ ertapenem sodium injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-3845 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERTAPENEM SODIUM (UNII: 2T90KE67L0) (ertapenem - UNII:G32F6EID2H) ertapenem 1 g Inactive Ingredients Ingredient Name Strength sodium bicarbonate (UNII: 8MDF5V39QO) 175 mg sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-3845-71 10 in 1 TRAY 11/21/2001 07/31/2018 1 NDC:0006-3845-01 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021337 11/21/2001 07/31/2018
CloseLabeler - Merck Sharp & Dohme LLC (118446553)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
INVANZ- ertapenem sodium injection, powder, lyophilized, for solution
Number of versions: 25
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Feb 19, 2024 | 38 (current) | download |
| Feb 8, 2024 | 37 | download |
| Jun 16, 2022 | 36 | download |
| Apr 4, 2022 | 35 | download |
| Jul 14, 2021 | 33 | download |
| Jan 21, 2021 | 32 | download |
| Mar 18, 2020 | 30 | download |
| Jun 10, 2019 | 29 | download |
| Jan 16, 2019 | 28 | download |
| Dec 13, 2018 | 27 | download |
| Oct 11, 2018 | 26 | download |
| May 4, 2018 | 24 | download |
| Aug 25, 2014 | 23 | download |
| Jul 17, 2013 | 21 | download |
| Mar 9, 2012 | 19 | download |
| Feb 21, 2012 | 18 | download |
| Dec 5, 2011 | 17 | download |
| Aug 12, 2011 | 15 | download |
| Aug 5, 2011 | 14 | download |
| Jun 4, 2010 | 13 | download |
| Jul 22, 2009 | 7 | download |
| Jul 25, 2008 | 4 | download |
| Mar 6, 2008 | 3 | download |
| Feb 9, 2007 | 2 | download |
| Aug 29, 2006 | 1 | download |
RxNorm
INVANZ- ertapenem sodium injection, powder, lyophilized, for solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1734683 | ertapenem sodium 1 GM Injection | PSN |
| 2 | 1734683 | ertapenem 1000 MG Injection | SCD |
| 3 | 1734683 | ertapenem 1 GM Injection | SY |
| 4 | 1734683 | ertapenem (as ertapenem sodium 1.046 GM) Injection | SY |
| 5 | 1734686 | INVANZ 1 GM Injection | PSN |
| 6 | 1734686 | ertapenem 1000 MG Injection [Invanz] | SBD |
| 7 | 1734686 | Invanz 1000 MG Injection | SY |
| 8 | 1734686 | Invanz 1 GM Injection | SY |
Get Label RSS Feed for this Drug
INVANZ- ertapenem sodium injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=33f3b99b-fa82-42e0-26bf-f49891ae3d22
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
INVANZ- ertapenem sodium injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0006-3843-71 |
| 2 | 0006-3845-01 |
| 3 | 0006-3845-71 |