Label: INFLATHERM- diclofenac sodium and menthol / camphor gel kit
- NDC Code(s): 0228-2551-06, 61577-3234-5, 72835-701-02
- Packager: V2 Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (see WARNINGS).
- Diclofenac sodium delayed-release tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see CONTRAINDICATIONS, WARNINGS).
Gastrointestinal Bleeding, Ulceration, And Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (see WARNINGS).
-
DESCRIPTION
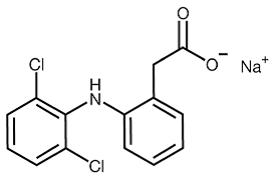
Diclofenac sodium delayed-release tablets, USP are a benzene-acetic acid derivative. Diclofenac sodium, USP is a white to almost white crystalline powder and is sparingly soluble in water at 25°C ...
-
CLINICAL PHARMACOLOGY
Mechanism of Action - Diclofenac has analgesic, anti-inflammatory, and antipyretic properties. The mechanism of action of diclofenac sodium delayed-release, like that of other NSAIDs, is not ...
-
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of diclofenac sodium delayed-release tablets and other treatment options before deciding to use diclofenac sodium delayed-release tablets. Use ...
-
CONTRAINDICATIONS
Diclofenac sodium delayed-release tablets are contraindicated in the following patients: Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to diclofenac or any ...
-
WARNINGS
Cardiovascular - Thrombotic Events - Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV ...
-
PRECAUTIONS
General - Diclofenac sodium delayed-release tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may ...
-
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling: Cardiovascular Thrombotic Events (see WARNINGS) GI Bleeding, Ulceration and Perforation (see ...
-
OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care ...
-
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of diclofenac sodium delayed-release tablets and other treatment options before deciding to use diclofenac sodium delayed-release tablets. Use ...
-
HOW SUPPLIED
Diclofenac Sodium Delayed-Release Tablets, USP are available as follows: 50 mg — Each white, round, enteric-coated tablet printed with on one side and 550 on the other side with black ink ...
-
Medication Guide for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)? NSAIDs can cause serious side effects, including: Increased risk ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 0228-2551-06 - Diclofenac Sodium Delayed-Release Tablets, USP - 75 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Actavis - 60 Enteric-Coated Tablets - Rx Only
- Sore No More Warm Therapy
-
Active IngredientsMenthol USP 3%, Camphor USP 3%
-
PurposePurpose - External Analgesic
-
Keep out of reach of childrenKeep out of reach of children
-
UsesTemporarily relieves minor aches and pains of muscles and joints associated with: arthritis, simple backaches
-
WarningsFor external use only. Do not use on wounds or damaged skin. When using this product: avoid bandaging tightly, avoid contact with eyes, keep out of reach of children. Stop use and ask doctor ...
-
DirectionsAdults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily, rub in thoroughly until gel is absorbed, children under 2 years of age: consult a ...
-
Inactive Ingredientsaloe barbadensis leaf juice, carbomer, decyl glucoside, water, citrus grandis (grapefruit) seed extract, camellia sinensis (green tea) leaf extract, citrus aurantium dulcis (orange) peel oil ...
-
Questions or Comments1-800-225-3963
-
Sore No More inner packaging

-
Inflatherm PackagingThe packaging for Inflatherm is shown below
-
INGREDIENTS AND APPEARANCEProduct Information


 on one side and 550 on the other side with black ink contains 50 mg of Diclofenac Sodium, USP. Tablets are supplied in bottles of 60 (NDC 0228-2550-06), 100 (NDC 0228-2550-11) and 1000 (NDC 0228-2550-96).
on one side and 550 on the other side with black ink contains 50 mg of Diclofenac Sodium, USP. Tablets are supplied in bottles of 60 (NDC 0228-2550-06), 100 (NDC 0228-2550-11) and 1000 (NDC 0228-2550-96). on one side and 551 on the other side with black ink contains 75 mg of Diclofenac Sodium, USP. Tablets are supplied in bottles of 60 (NDC 0228-2551-06), 100 (NDC 0228-2551-11), and 1000 (NDC 0228-2551-96).
on one side and 551 on the other side with black ink contains 75 mg of Diclofenac Sodium, USP. Tablets are supplied in bottles of 60 (NDC 0228-2551-06), 100 (NDC 0228-2551-11), and 1000 (NDC 0228-2551-96).
