Label: INCRELEX- mecasermin injection, solution
- NDC Code(s): 15054-1040-5
- Packager: Ipsen Biopharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INCRELEX® safely and effectively. See full prescribing information for INCRELEX®. INCRELEX® (mecasermin) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESevere Primary IGF-1 Deficiency (Primary IGFD) INCRELEX is indicated for the treatment of growth failure in pediatric patients 2 years of age and older with: severe primary IGF-1 deficiency ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Treatment with INCRELEX should be supervised by a physician who is experienced in the diagnosis and management of pediatric patients with short stature associated with ...
-
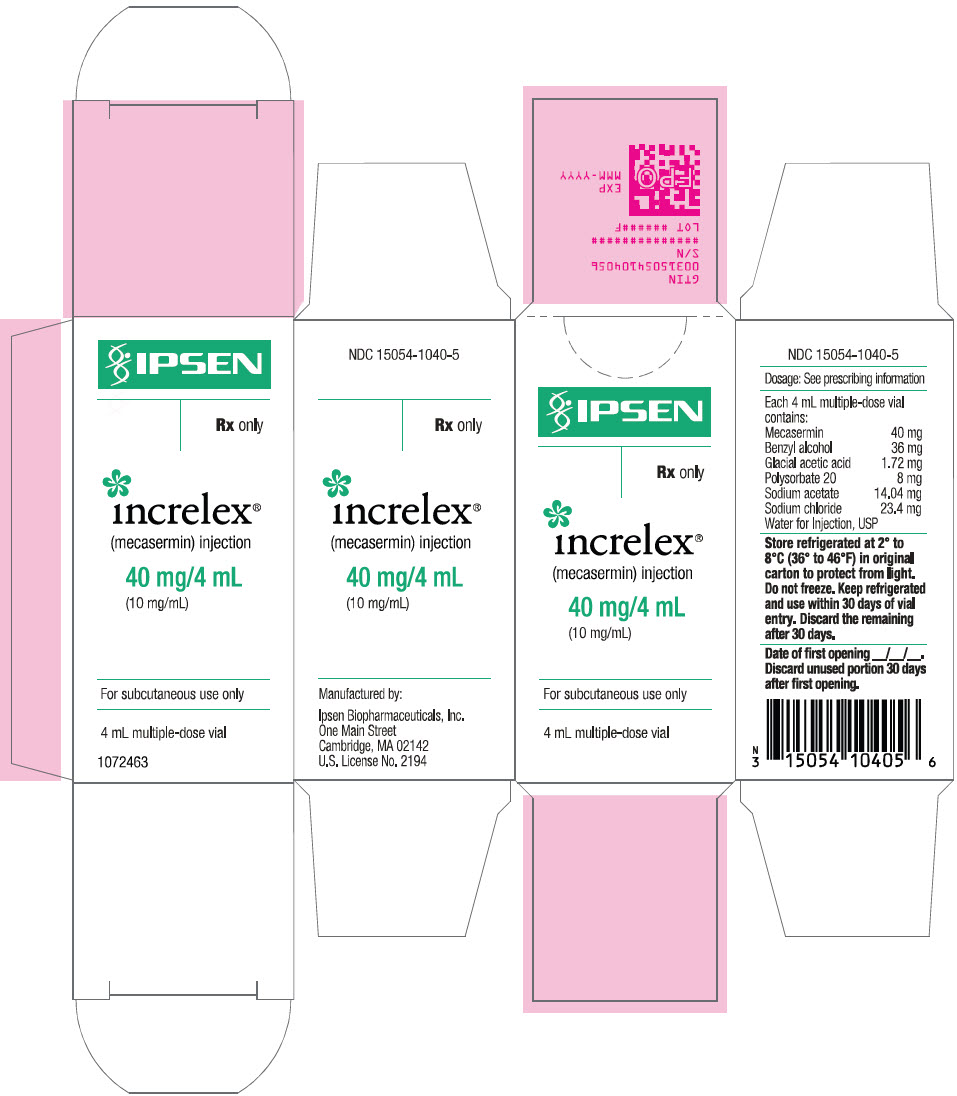
3 DOSAGE FORMS AND STRENGTHSInjection: 40 mg/4 mL (10 mg/mL) clear and colorless solution in a multiple-dose glass vial.
-
4 CONTRAINDICATIONSKnown Hypersensitivity - INCRELEX should not be used by patients who are allergic to mecasermin (rhIGF-1) or any of the inactive ingredients in INCRELEX, or who have experienced a severe ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - Severe hypolgycemia leading to hypolycemic seizures has been observed with INCRELEX treatment [see Adverse Reactions (6.1)]. Because INCRELEX has insulin-like hypoglycemic ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions (5.1)]. Hypersensitivity and Allergic Reactions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on INCRELEX use in pregnant women. Exposure to INCRELEX during pregnancy is unlikely because the drug is not indicated for use after ...
-
10 OVERDOSAGETreatment of acute overdose should be directed at reversing hypoglycemia. Oral glucose or food should be consumed. If the overdose results in loss of consciousness, intravenous glucose or ...
-
11 DESCRIPTIONMecasermin is a human insulin-like growth factor-1 (rhIGF-1) produced by recombinant DNA technology. IGF-1 consists of 70 amino acids in a single chain with three intramolecular disulfide bridges ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Insulin-like growth factor-1 (IGF-1) is a key hormonal mediator on statural growth. Under normal circumstances, growth hormone (GH) binds to its receptor in the liver ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: INCRELEX was tumorigenic in rats in a study using doses of 0, 0.25, 1, 4, and 10 mg/kg/day by subcutaneous ...
-
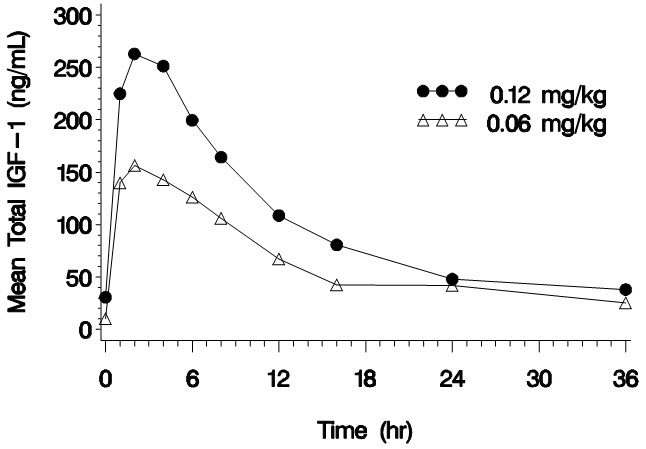
14 CLINICAL STUDIES14.1 Effects of INCRELEX Treatment in Children with Severe Primary Insulin-like Growth Factor-1 Deficiency (Severe Primary IGFD) Five clinical studies (four open-label and one double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - INCRELEX injection is supplied as a 40 mg/4 mL (10 mg/mL) sterile, clear, and colorless solution in multiple-dose glass vials (NDC-15054-1040-5). Storage and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients and/or caregivers to read the FDA-approved patient labeling (Patient Information) and Instructions for Use. Counsel patients and/or parents that there have been occurrences of ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Ipsen Biopharmaceuticals, Inc. Cambridge, MA 02142 USA - U.S. License No. 2194
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationRevised: 10/2023 - PATIENT INFORMATION - INCRELEX® (EENK-RUH-LEX) (mecasermin) injection - for subcutaneous ...
-
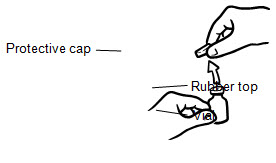
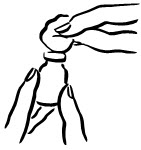
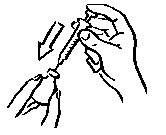
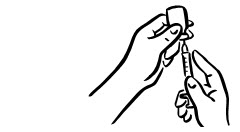
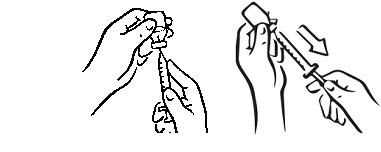
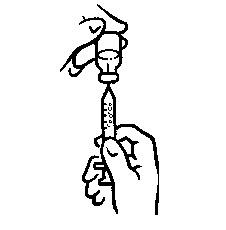
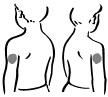
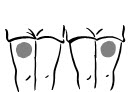
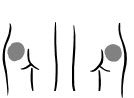
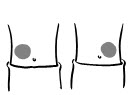
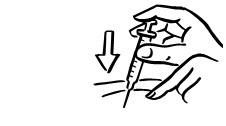
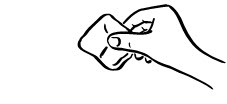
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - INCRELEX® (EENK-RUH-LEX) (mecasermin) injection - for subcutaneous use - Read this Instructions for Use before you start using INCRELEX and each time you get a refill. There may ...
-
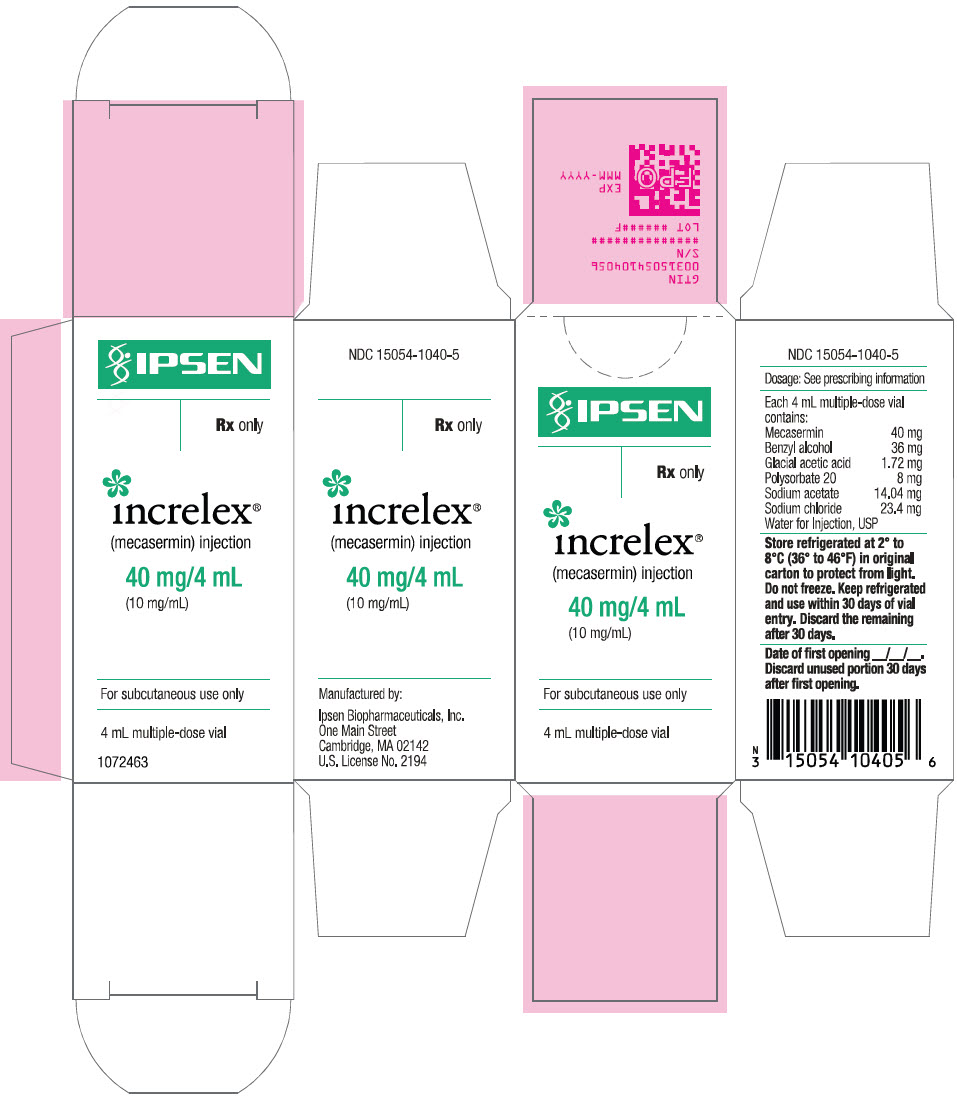
PRINCIPAL DISPLAY PANEL - 4 mL Vial CartonIPSEN - Rx only - increlex® (mecasermin) injection - 40 mg/4 mL - (10 mg/mL) For subcutaneous use only - 4 mL multiple-dose vial
-
INGREDIENTS AND APPEARANCEProduct Information