Label: INBRIJA- levodopa capsule
- NDC Code(s): 10144-342-01, 10144-342-04, 10144-342-12, 10144-342-16, view more
- Packager: Merz Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INBRIJA safely and effectively. See full prescribing information for INBRIJA. INBRIJA - ® (levodopa inhalation powder), for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEINBRIJA is indicated for the intermittent treatment of OFF episodes in patients with Parkinson's disease treated with carbidopa/levodopa.
-
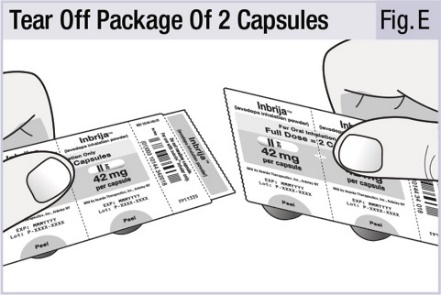
2 DOSAGE AND ADMINISTRATIONINBRIJA capsules are for oral inhalation only and should be used only with the INBRIJA inhaler. 2.1 Important Administration Instructions - INBRIJA capsules are for oral inhalation only and ...
-
3 DOSAGE FORMS AND STRENGTHSINBRIJA (levodopa inhalation powder) consists of INBRIJA capsules and the INBRIJA inhaler. INBRIJA capsules contain 42 mg dry powder formulation of levodopa in a white capsule with two black color ...
-
4 CONTRAINDICATIONSINBRIJA is contraindicated in patients currently taking a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine and tranylcypromine) or who have recently (within 2 weeks) taken a ...
-
5 WARNINGS AND PRECAUTIONS5.1 Falling Asleep During Activities of Daily Living and Somnolence - Patients treated with levodopa, the active ingredient in INBRIJA, have reported falling asleep while engaged in activities ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed below and elsewhere in the labeling: Falling Asleep During Activities of Daily Living and Somnolence - [see ...
-
7 DRUG INTERACTIONS7.1 Monoamine Oxidase (MAO) Inhibitors - The use of nonselective MAO inhibitors with INBRIJA is contraindicated - [see - Contraindications (4)] ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of INBRIJA in pregnant women. In animal studies, carbidopa/levodopa has been shown ...
-
10 OVERDOSAGEBased on the limited available information, the acute symptoms of carbidopa/levodopa overdosage can be expected to arise from dopaminergic overstimulation. Using more than one dose (84 mg) to ...
-
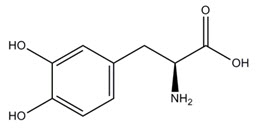
11 DESCRIPTIONINBRIJA consists of a dry powder formulation of levodopa for oral inhalation with the INBRIJA inhaler. The inhalation powder is packaged in white hypromellose capsules. Each capsule contains a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Levodopa, the metabolic precursor of dopamine, crosses the blood-brain barrier and presumably is converted to dopamine in the brain. This is thought to be the mechanism ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In rats, oral administration of carbidopa/levodopa for two years resulted in no evidence of ...
-
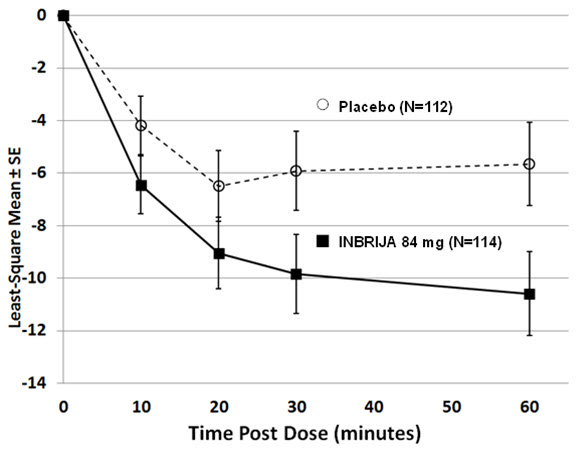
14 CLINICAL STUDIESThe efficacy and safety of INBRIJA for the treatment of OFF episodes in patients with Parkinson's disease treated with oral carbidopa/levodopa was evaluated in a 12-week, randomized ...
-
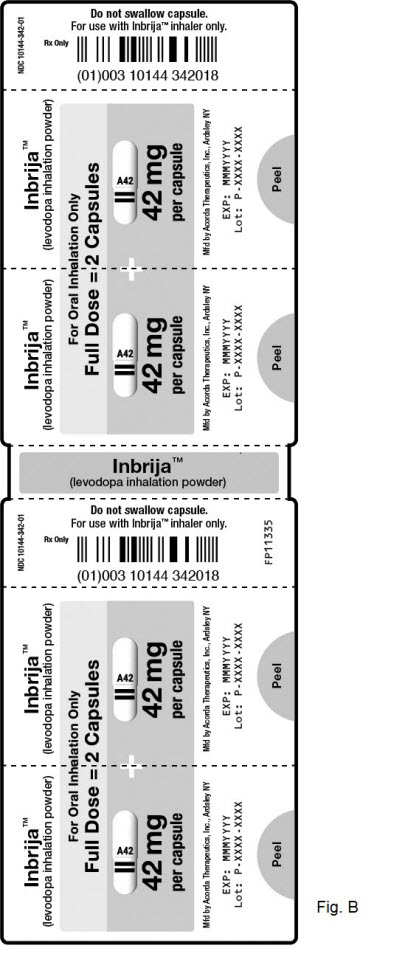
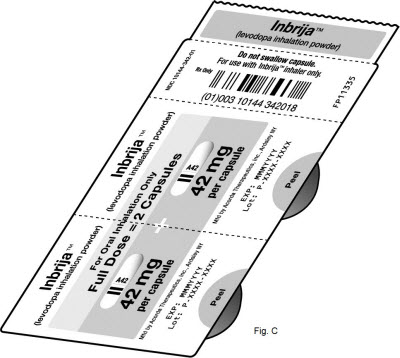
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - INBRIJA 42 mg contains foil blister strips of INBRIJA (levodopa inhalation powder) white capsules with two black bands on the body and "A42" in black on the cap, and one ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Instructions for Administering INBRIJA - It is important for patients to ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Acorda Therapeutics, Inc. Pearl River, NY 10965 USA - 2212342WPI-0
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationIssued: 12/2022 - PATIENT INFORMATION - INBRIJA - ® (in-BRIH-jah) (levodopa ...
-
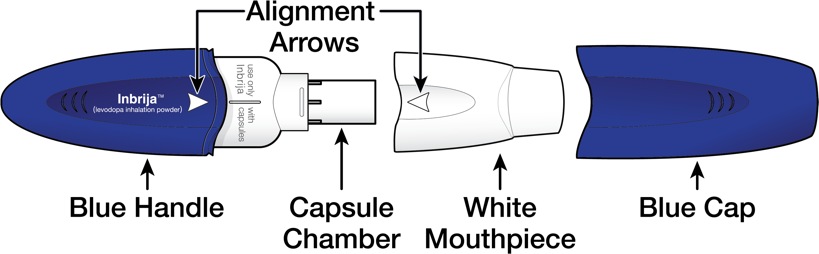
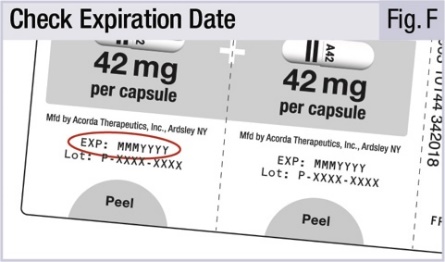
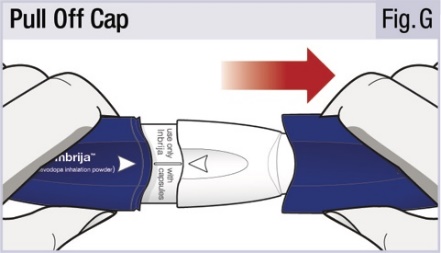
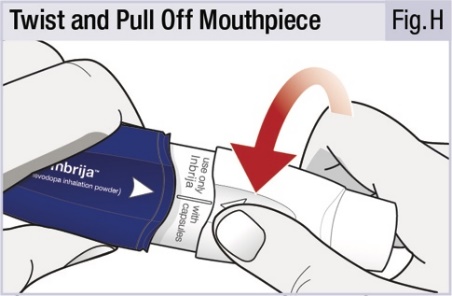
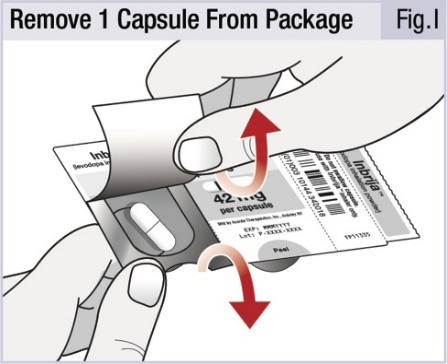
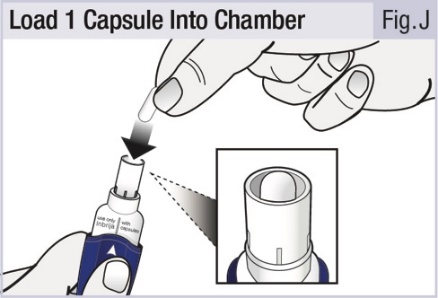
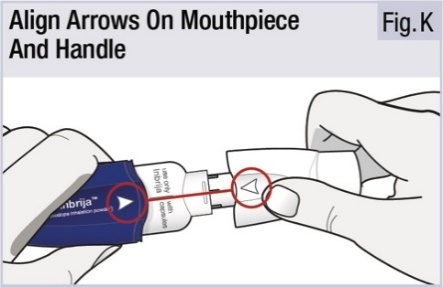
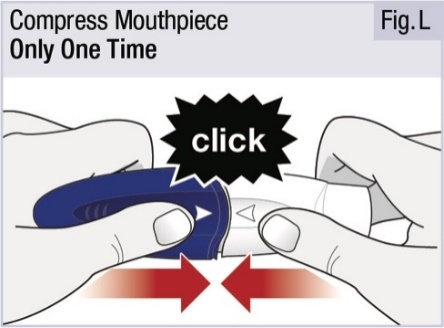
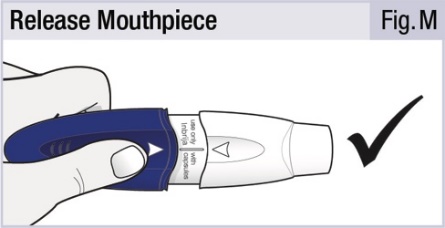
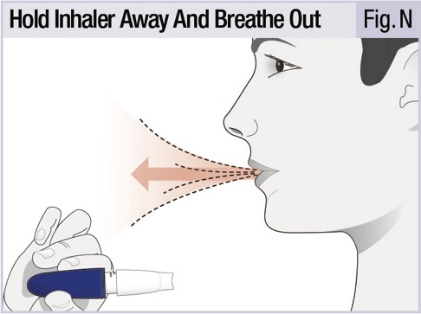
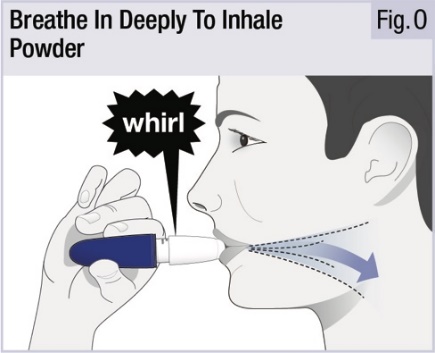
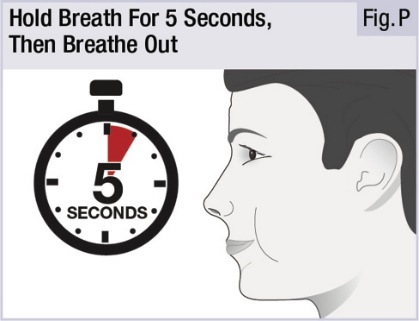
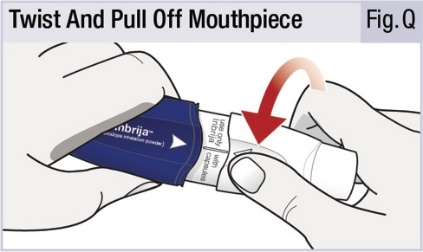
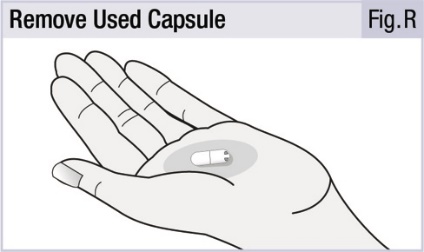
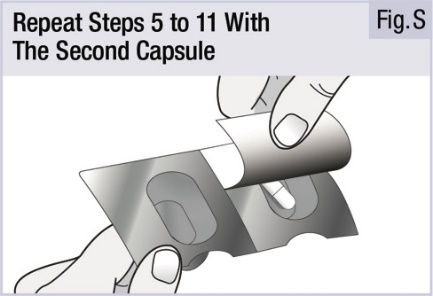
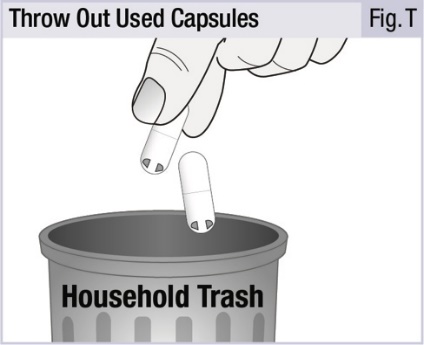
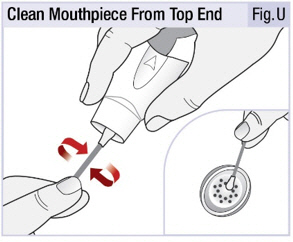
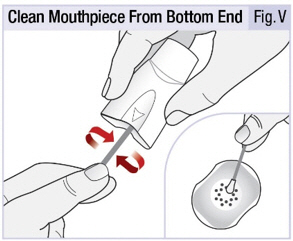
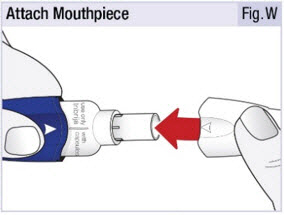
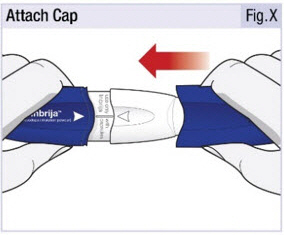
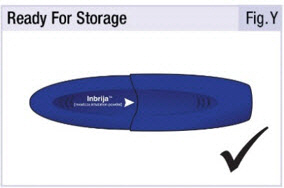
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - INBRIJA - ® (in-BRIH-jah) (levodopa inhalation powder) For Oral Inhalation Only - Read and follow these instructions before you start using INBRIJA and each ...
-
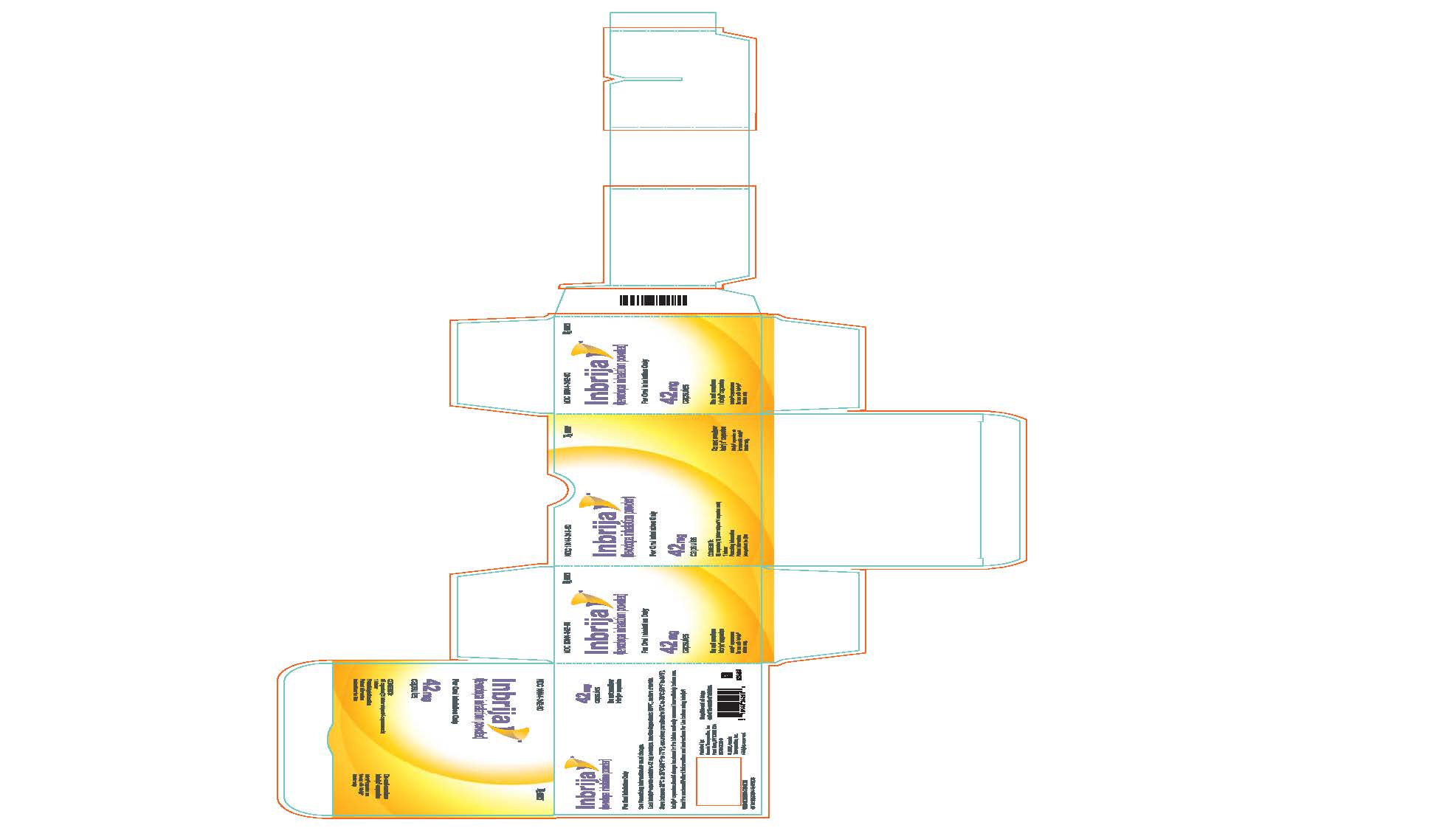
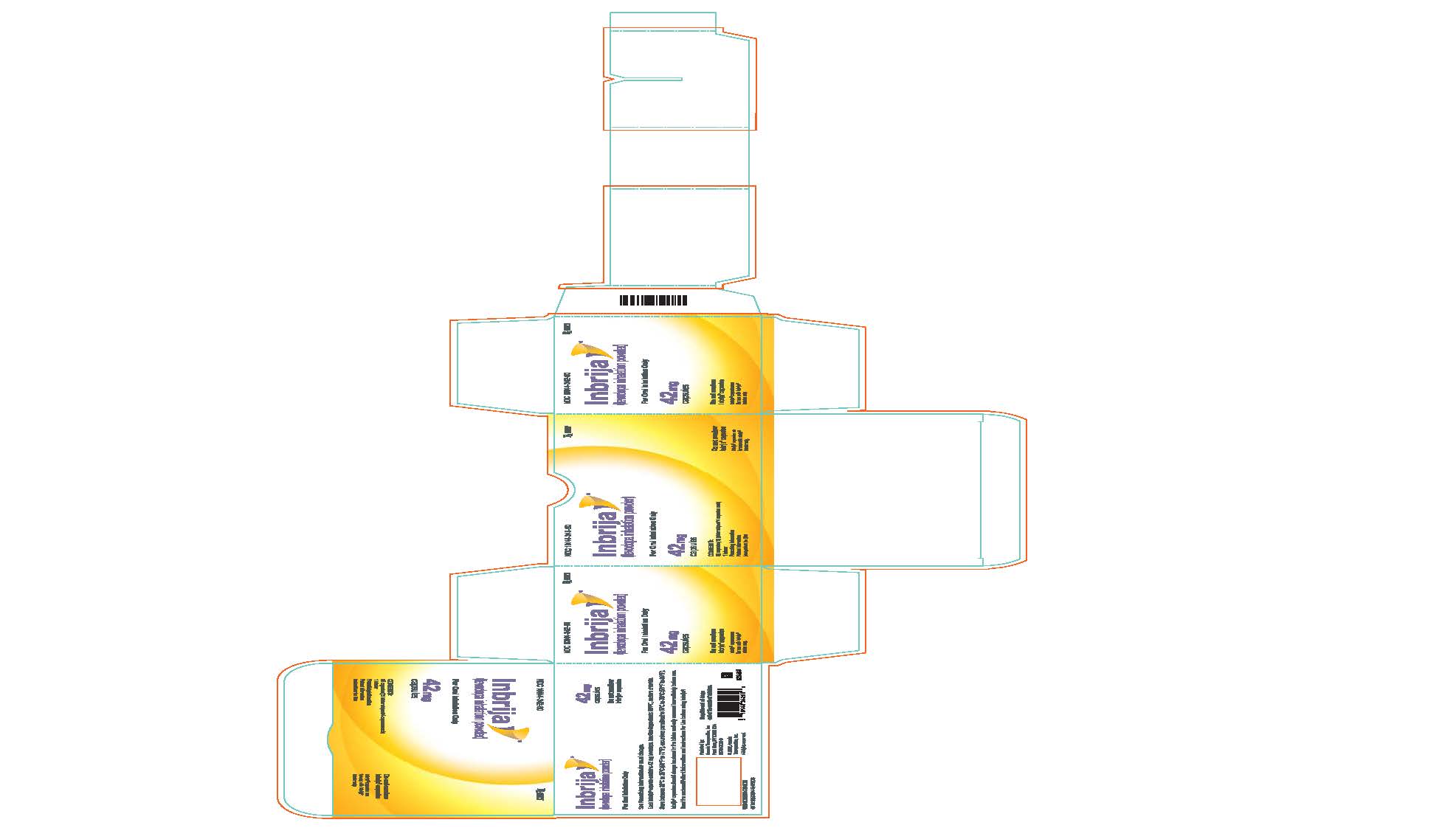
PRINCIPAL DISPLAY PANEL - 42 mg Capsule Blister Pack CartonNDC 10144-342-60 - Rx ONLY - Inbrija® (levodopa inhalation powder) For Oral Inhalation Only - 42 mg - capsules - CONTENTS: 60 capsules (15 blister - strips of 4 capsules ...
-
INGREDIENTS AND APPEARANCEProduct Information