Label: IDAMYCIN PFS- idarubicin hydrochloride solution
- NDC Code(s): 0013-2576-05, 0013-2576-91, 0013-2586-10, 0013-2586-91, view more
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR INTRAVENOUS USE ONLY
-
BOXED WARNING
(What is this?)
WARNINGS
- 1.
- IDAMYCIN PFS Injection should be given slowly into a freely flowing intravenous infusion. It must never be given intramuscularly or subcutaneously. Severe local tissue necrosis can occur if there is extravasation during administration.
- 2.
- As is the case with other anthracyclines the use of IDAMYCIN PFS can cause myocardial toxicity leading to congestive heart failure. Cardiac toxicity is more common in patients who have received prior anthracyclines or who have pre-existing cardiac disease.
- 3.
- As is usual with antileukemic agents, severe myelosuppression occurs when IDAMYCIN PFS is used at effective therapeutic doses.
- 4.
- It is recommended that IDAMYCIN PFS be administered only under the supervision of a physician who is experienced in leukemia chemotherapy and in facilities with laboratory and supportive resources adequate to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The physician and institution must be capable of responding rapidly and completely to severe hemorrhagic conditions and/or overwhelming infection.
- 5.
- Dosage should be reduced in patients with impaired hepatic or renal function. (See DOSAGE AND ADMINISTRATION.)
-
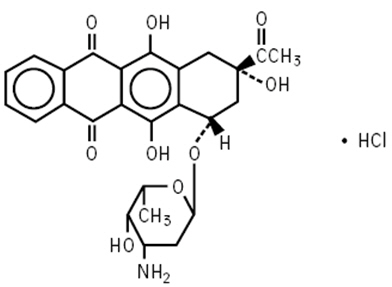
DESCRIPTIONIDAMYCIN PFS Injection contains idarubicin hydrochloride and is a sterile, semi-synthetic, preservative-free solution (PFS) antineoplastic anthracycline for intravenous use. Chemically, idarubicin ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Idarubicin hydrochloride is a DNA-intercalating analog of daunorubicin which has an inhibitory effect on nucleic acid synthesis and interacts with the enzyme topoisomerase ...
-
CLINICAL STUDIESFour prospective randomized studies, three U.S. and one Italian, have been conducted to compare the efficacy and safety of idarubicin (IDR) to that of daunorubicin (DNR), each in combination with ...
-
INDICATIONS AND USAGEIDAMYCIN PFS Injection in combination with other approved antileukemic drugs is indicated for the treatment of acute myeloid leukemia (AML) in adults. This includes French-American-British (FAB ...
-
WARNINGSIdarubicin is intended for administration under the supervision of a physician who is experienced in leukemia chemotherapy. Idarubicin is a potent bone marrow suppressant. Idarubicin should not be ...
-
PRECAUTIONSGeneral - Therapy with idarubicin requires close observation of the patient and careful laboratory monitoring. Hyperuricemia secondary to rapid lysis of leukemic cells may be induced. Appropriate ...
-
ADVERSE REACTIONSApproximately 550 patients with AML have received idarubicin in combination with cytarabine in controlled clinical trials worldwide. In addition, over 550 patients with acute leukemia have been ...
-
OVERDOSAGEThere is no known antidote to idarubicin. Two cases of fatal overdosage in patients receiving therapy for AML have been reported. The doses were 135 mg/m2 over 3 days and 45 mg/m2 of idarubicin ...
-
DOSAGE AND ADMINISTRATION(See WARNINGS) For induction therapy in adult patients with AML the following dose schedule is recommended: IDAMYCIN PFS Injection 12 mg/m2 daily for 3 days by slow (10 to 15 min) intravenous ...
-
Handling and DisposalProcedures for handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1–8 There is no general agreement that all of the procedures ...
-
HOW SUPPLIEDIDAMYCIN PFS Injection (idarubicin hydrochloride injection) is a clear, orange-red, aqueous, preservative-free solution, free from visible particles containing 1 mg/mL idarubicin ...
-
REFERENCES1. ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society. 1999: 32–41. 2. Recommendations for the Safe ...
-
SPL UNCLASSIFIED SECTION

-
SPL UNCLASSIFIED SECTIONLAB-0131-9.0 - Revised November 2023
-
PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Vial LabelNDC 0013-2576-91 - 5 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 5 mg/5 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution - Rx only
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial CartonNDC 0013-2576-91 - 5 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 5 mg/5 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution - Pfizer - Hospital - Rx ...
-
PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Glass Vial Label NDC 0013-2576-05 - 5 mL Single-Dose Vial - Idamycin PFS® idarubicin hydrochloride injection - 5 mg/5 mL - (1 mg/1 mL) For Intravenous Use Only - Caution: Cytotoxic Agent - Sterile: Isotonic Solution - Rx ...
-
PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Glass Vial Carton NDC 0013-2576-05 - 5 mL Single-Dose Vial - Discard unused portion - Idamycin PFS® idarubicin hydrochloride - injection - 5 mg/5 mL - (1 mg/mL) For Intravenous Use Only - Sterile, Isotonic ...
-
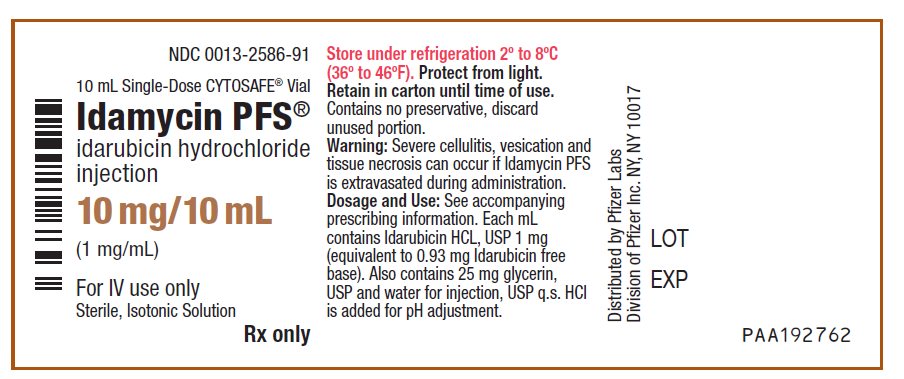
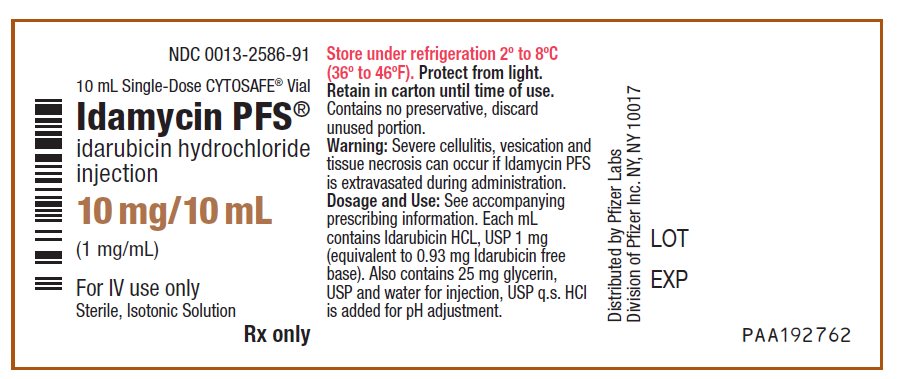
PRINCIPAL DISPLAY PANEL - 10 mg/10 mL Vial LabelNDC 0013-2586-91 - 10 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 10 mg/10 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution - Rx only
-
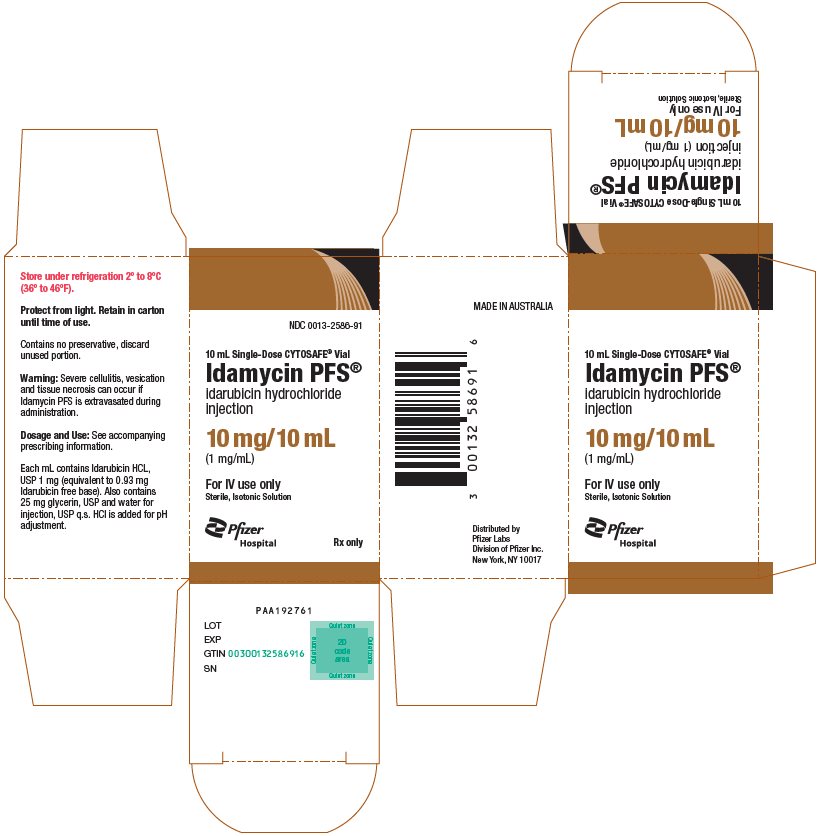
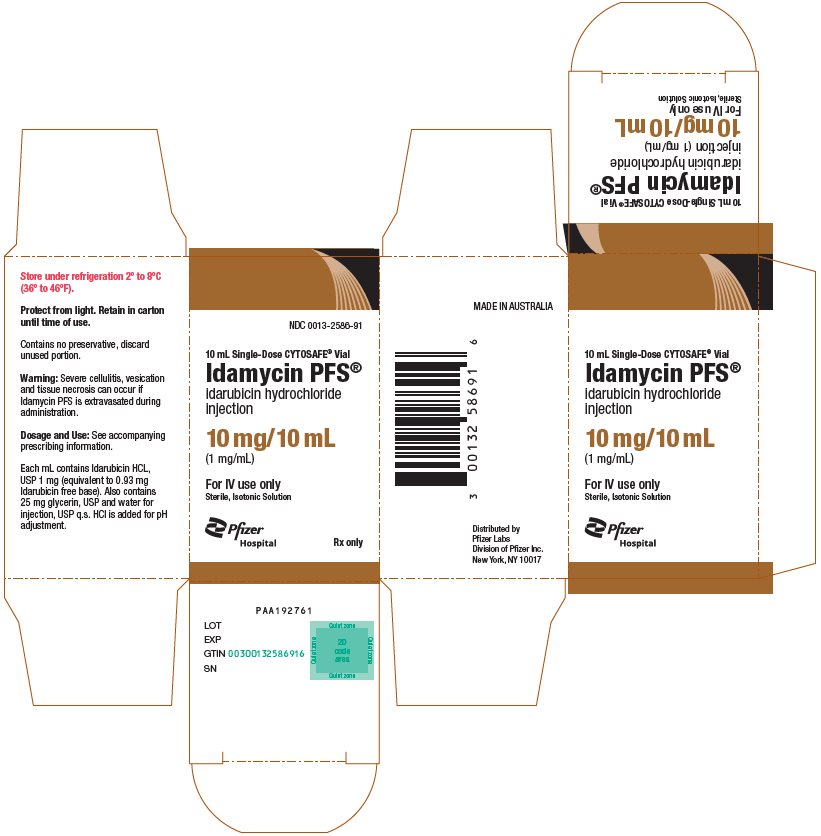
PRINCIPAL DISPLAY PANEL - 10 mL Vial CartonNDC 0013-2586-91 - 10 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 10 mg/10 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution - Pfizer - Hospital - Rx ...
-
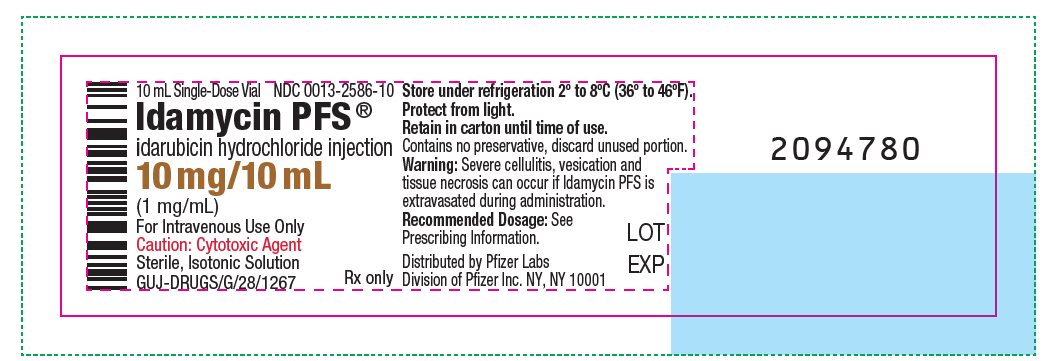
PRINCIPAL DISPLAY PANEL - 10 mg/10 mL Glass Vial Label 10 mL Single- Dose Vial - NDC 0013-2586-10 - Idamycin PFS® idarubicin hydrochloride injection - 10 mg/10 mL - (1 mg/mL) For Intravenous Use Only - Caution: Cytotoxic Agent - Sterile, Isotonic ...
-
PRINCIPAL DISPLAY PANEL - 10 mg/10 mL Glass Vial Carton NDC 0013-2586-10 - 10 mL Single-Dose Vial - Discard unused portion - Idamycin PFS® idarubicin hydrochloride - injection - 10 mg/10 mL - (1 mg/mL) For Intravenous Use Only - Sterile, Isotonic Solution - Pfizer ...
-
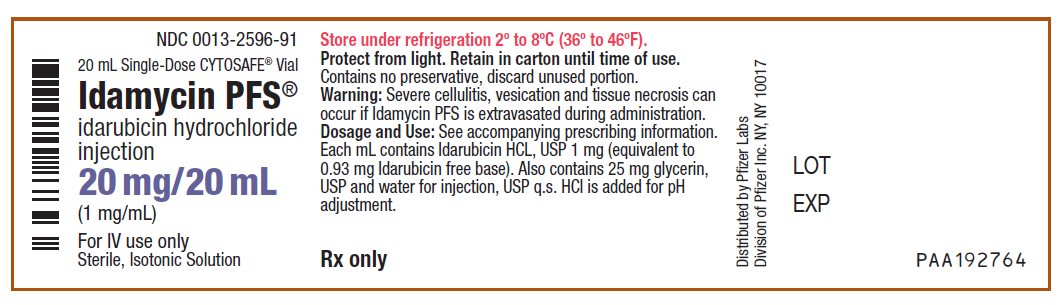
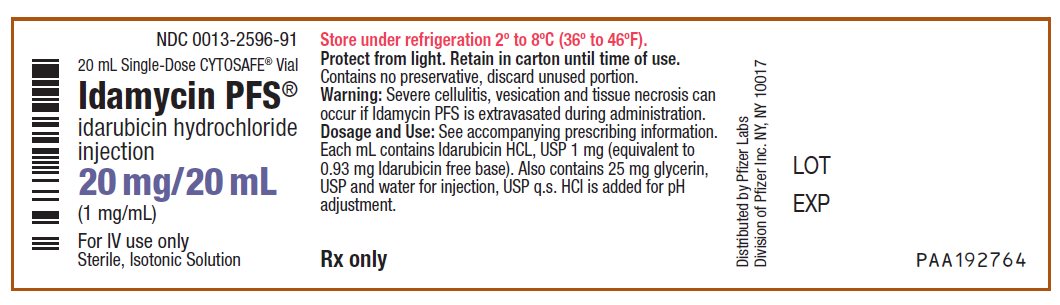
PRINCIPAL DISPLAY PANEL - 20 mg/20 mL Vial LabelNDC 0013-2596-91 - 20 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 20 mg/20 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution
-
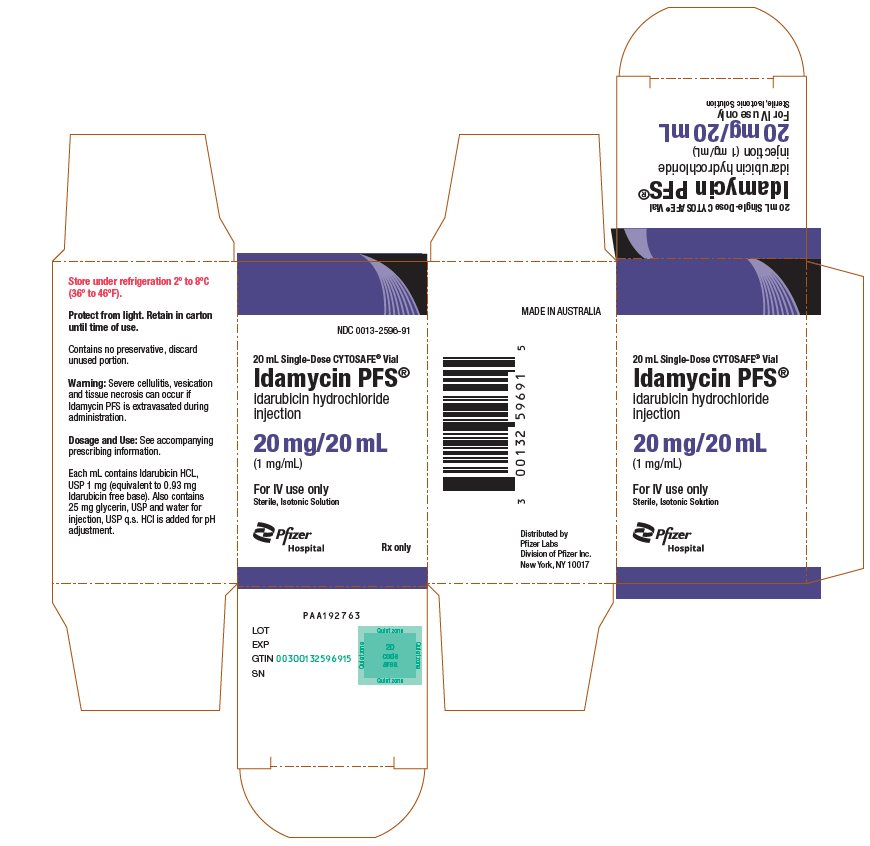
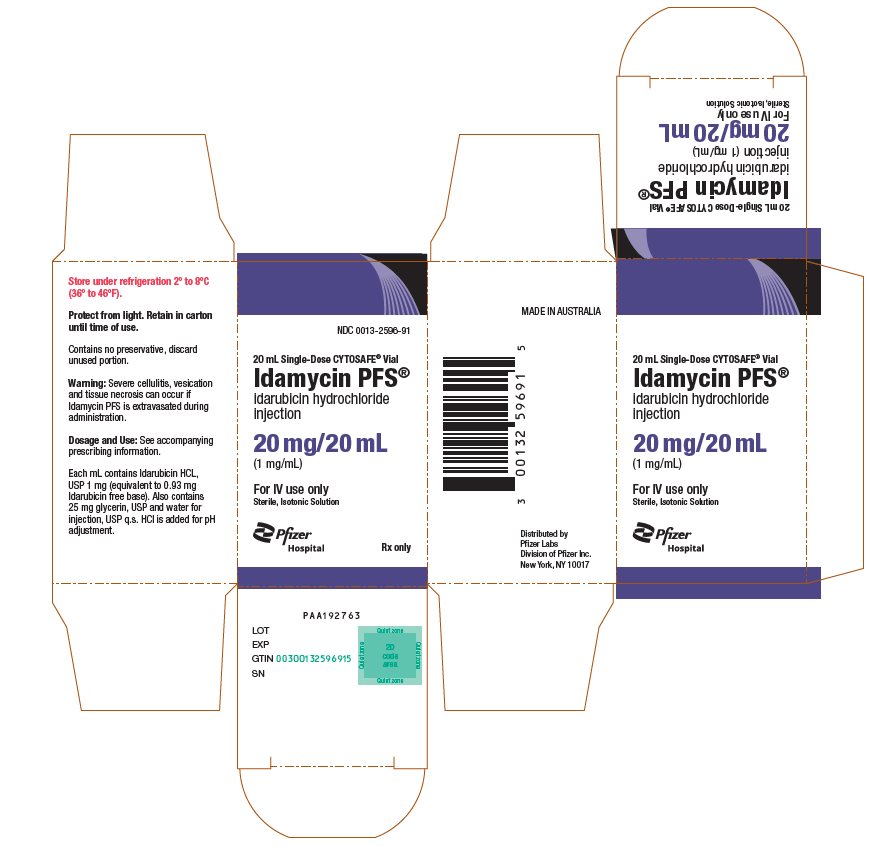
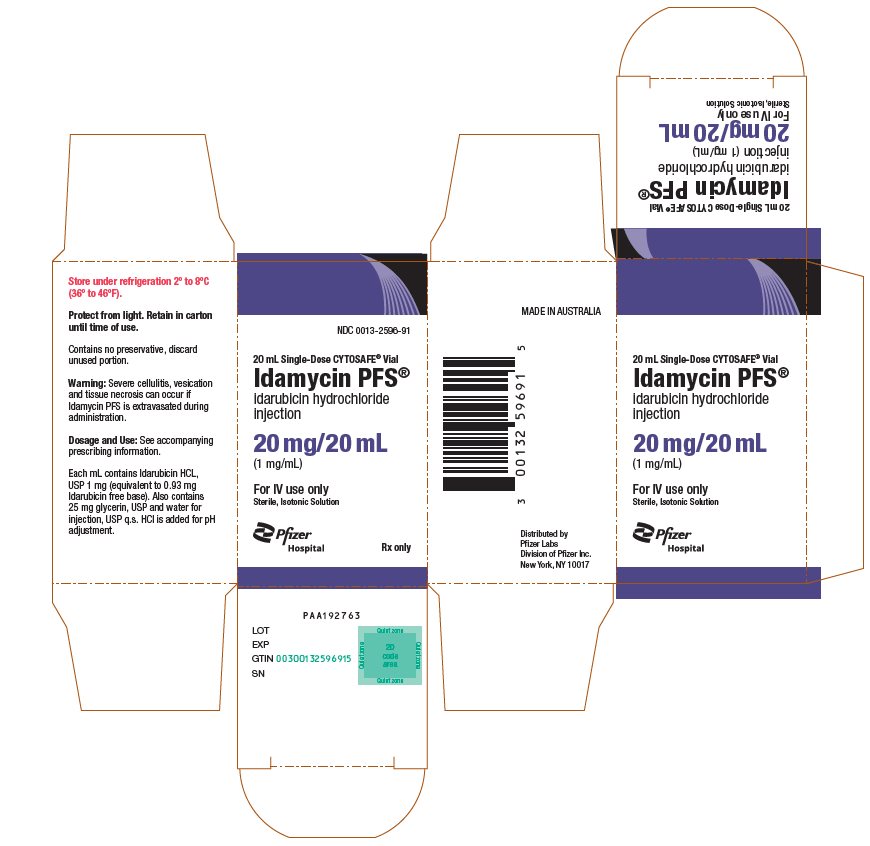
PRINCIPAL DISPLAY PANEL - 20 mL Vial CartonNDC 0013-2596-91 - 20 mL Single-Dose CYTOSAFE® Vial - Idamycin PFS® idarubicin hydrochloride - injection - 20 mg/20 mL - (1 mg/mL) For IV use only - Sterile, Isotonic Solution - Pfizer - Hospital - Rx ...
-
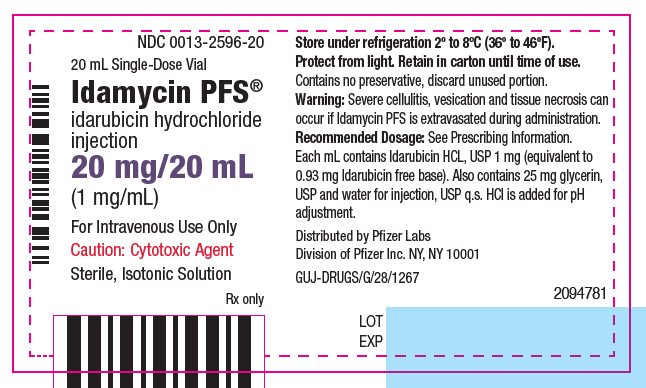
PRINCIPAL DISPLAY PANEL - 20 mg/20 mL Glass Vial Label NDC 0013-2596-20 - 20 mL Single-Dose Vial - Idamycin PFS® idarubicin hydrochloride - injection - 20 mg/20 mL - (1 mg/mL) For Intravenous Use Only - Caution: Cytotoxic Agent - Sterile, Isotonic Solution - Rx only ...
-
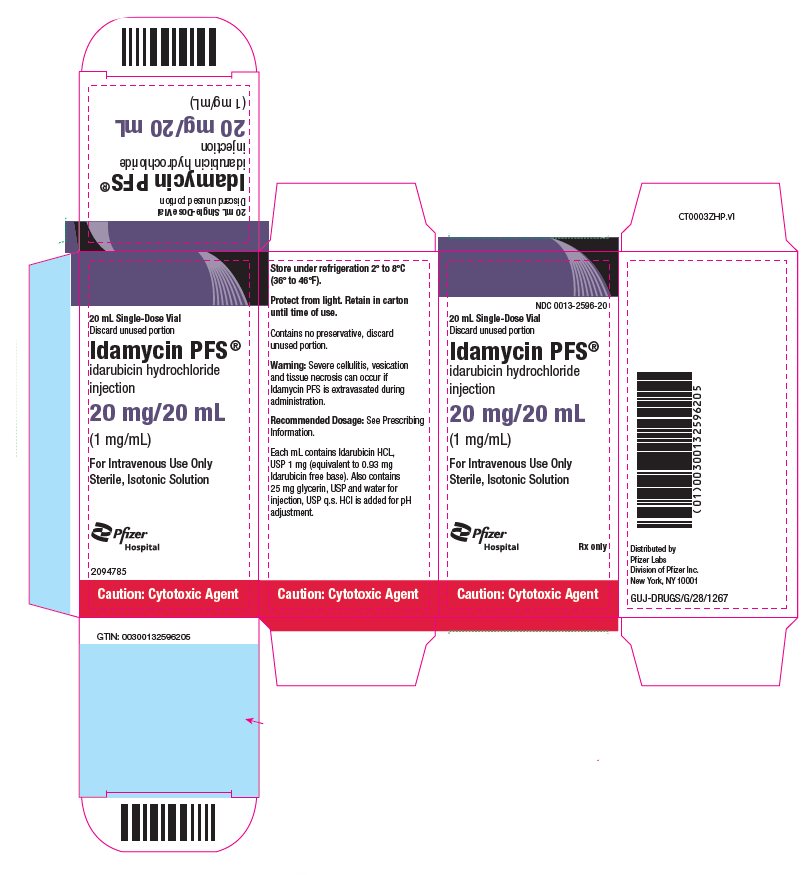
PRINCIPAL DISPLAY PANEL - 20 mg/20 mL Glass Vial Carton NDC 0013-2596-20 - 20 mL Single-Dose Vial - Discard unused portion - Idamycin PFS® idarubicin hydrochloride - injection - 20 mg/20 mL - (1 mg/mL) For Intravenous Use Only - Sterile, Isotonic ...
-
INGREDIENTS AND APPEARANCEProduct Information