Label: IBUPAK- ibuprofen kit
- NDC Code(s): 59088-756-00, 67877-320-01
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
Cardiovascular Thrombotic Events
· Non-steroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (see WARNINGS and PRECAUTIONS).
· Ibuprofen Tablets is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see CONTRAINDICATIONS and WARNINGS).
Gastrointestinal Risk
NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
Close -
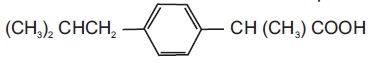
DESCRIPTIONIbuprofen tablets contain the active ingredient ibuprofen, which is (±)-2-( p-isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77° C and is very slightly ...
-
CLINICAL PHARMACOLOGYIbuprofen tablets contain ibuprofen which possesses analgesic and antipyretic activities. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to ...
-
INDICATIONS AND USAGECarefully consider the potential benefits and risks of ibuprofen tablets and other treatment options before deciding to use ibuprofen. Use the lowest effective dose for the shortest duration ...
-
CONTRAINDICATIONSIbuprofen tablets are contraindicated in patients with known hypersensitivity to ibuprofen. Ibuprofen tablets should not be given to patients who have experienced asthma, urticarial, or ...
-
WARNINGSCardiovascular Effects - Cardiovascular Thrombotic Events Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of ...
-
PRECAUTIONSGENERAL - Ibuprofen tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease ...
-
ADVERSE REACTIONSThe most frequent type of adverse reaction occurring with ibuprofen tablets is gastrointestinal. In controlled clinical trials the percentage of patients reporting one or more gastrointestinal ...
-
OVERDOSAGEApproximately 1½ hours after the reported ingestion of from 7 to10 ibuprofen tablets (400 mg), a 19-month old child weighing 12 kg was seen in the hospital emergency room, apneic and cyanotic ...
-
DOSAGE AND ADMINISTRATIONCarefully consider the potential benefits and risks of ibuprofen tablets and other treatment options before deciding to use ibuprofen tablets. Use the lowest effective dose for the shortest ...
-
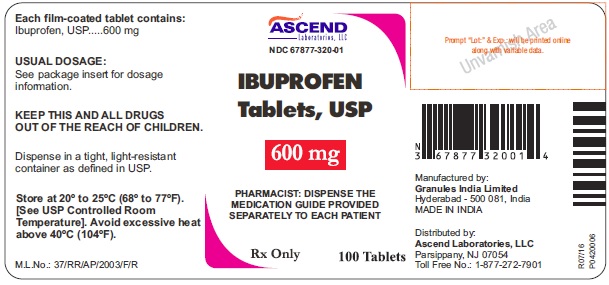
HOW SUPPLIED600 mg (white to off-white, caplet, 'I 7' debossing on one side and plain on the other side) Bottles of 100; NDC 67877-320-01 - Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled ...
-
SPL MEDGUIDEMedication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) (See the end of this Medication Guide for a list of prescription NSAID medicines.) What is the most important ...
-
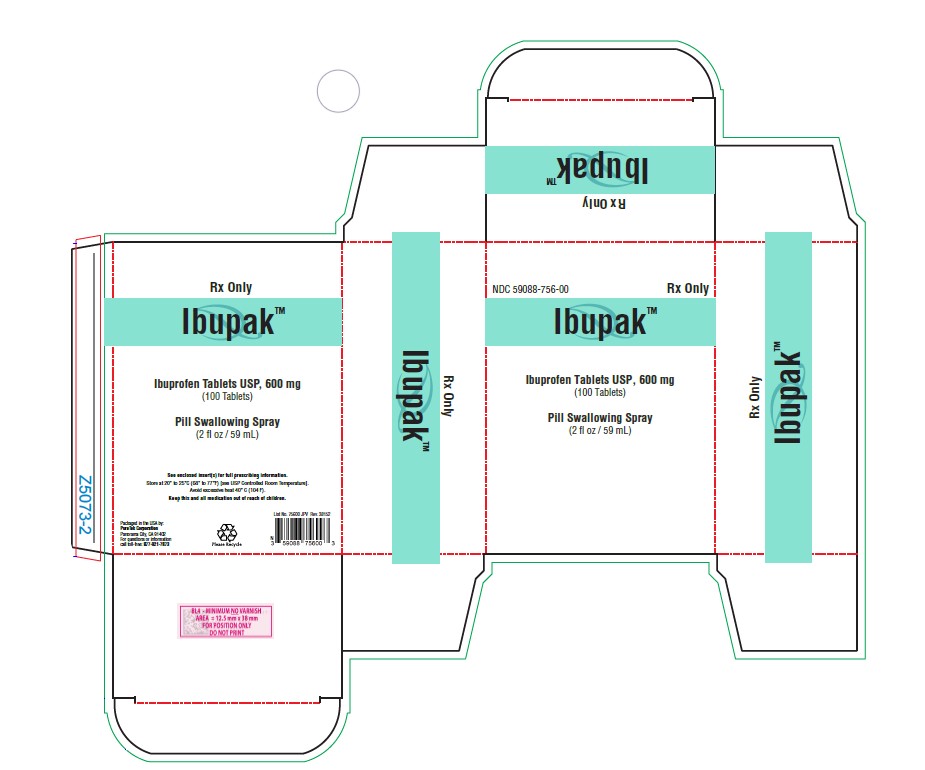
IBUPROFENNDC 67877-320-01 - Rx Only - PHARMACIST: DISPENSE THE MEDICATION GUIDE PROVIDED SEPARATELY TO EACH PATIENT
-
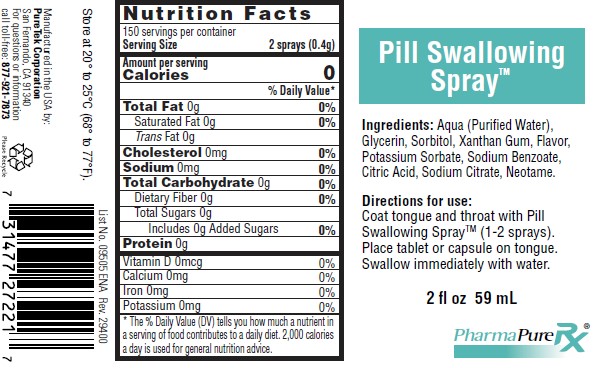
PHARMAPURERX® PILL SWALLOWING SPRAY™Directions for use: Coat tounge and throat with Pill Swallowing Spray™ (1-2 sprays). Place tablet or capsule on tounge. Swallow immediately with water. Ingredients: Aqua (Purified Water) ...
-
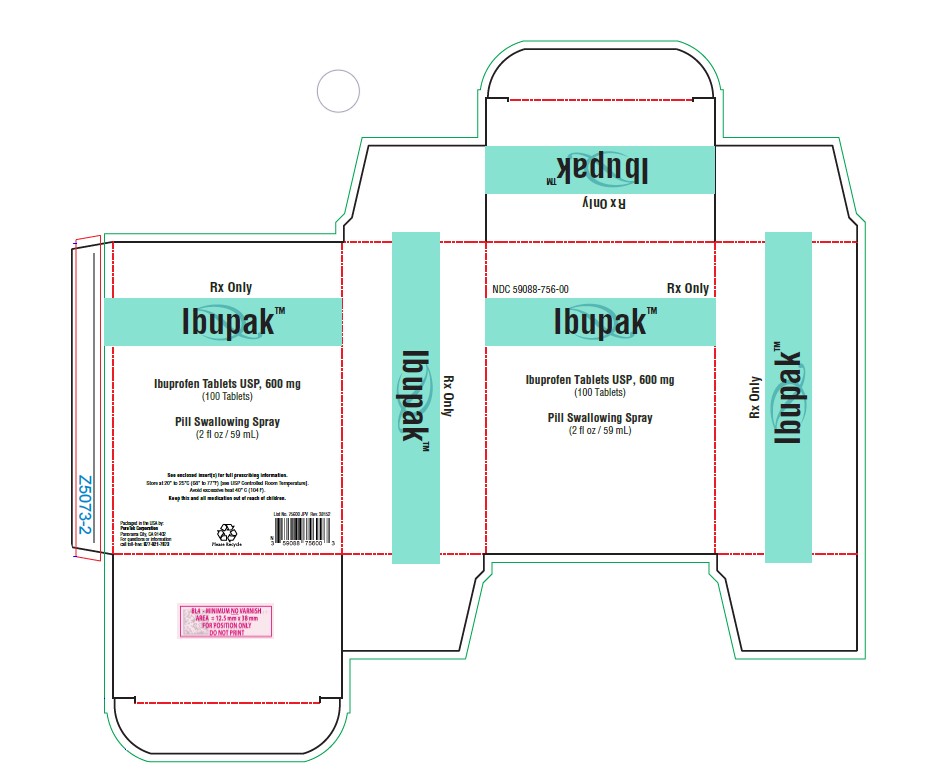
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPackaged in the USA by: PureTek Corporation - Panorama City, CA 91402 - For questions or information - call toll-free: 877-921-7873 - List No. 75600 JPV Rev. 38152
-
INGREDIENTS AND APPEARANCEProduct Information