Label: IBSRELA- tenapanor hydrochloride tablet
- NDC Code(s): 73154-050-06, 73154-050-60
- Packager: Ardelyx, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IBSRELA® safely and effectively. See full prescribing information for IBSRELA. IBSRELA (tenapanor) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
- IBSRELA is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile rats administration of tenapanor caused deaths presumed to be due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)].
- Avoid use of IBSRELA in patients 6 years to less than 12 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- The safety and effectiveness of IBSRELA have not been established in patients less than 18 years of age [see Use in Specific Populations (8.4)].
-
1 INDICATIONS AND USAGEIBSRELA is indicated for treatment of irritable bowel syndrome with constipation (IBS-C) in adults.
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of IBSRELA in adults is 50 mg orally twice daily. Administration Instructions - Take IBSRELA immediately prior to breakfast or the first meal of the day and immediately ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 50 mg tenapanor supplied as an oval, white to off-white tablet debossed with "50" on one side and "5791" on the other side.
-
4 CONTRAINDICATIONSIBSRELA is contraindicated in: Patients less than 6 years of age due to the risk of serious dehydration [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)]. Patients with ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Serious Dehydration in Pediatric Patients - IBSRELA is contraindicated in patients below 6 years of age. The safety and effectiveness of IBSRELA in patients less than 18 years of age ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 OATP2B1 Substrates - Tenapanor is an inhibitor of intestinal uptake transporter, OATP2B1 [see Clinical Pharmacology (12.3)]. Drugs which are substrates of OATP2B1 may have reduced exposures ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Tenapanor is minimally absorbed systemically, with plasma concentrations below the limit of quantification (less than 0.5 ng/mL) following oral administration ...
-
10 OVERDOSAGEBased on nonclinical data, overdose of IBSRELA may result in gastrointestinal adverse effects such as diarrhea as a result of exaggerated pharmacology with a risk for dehydration if diarrhea is ...
-
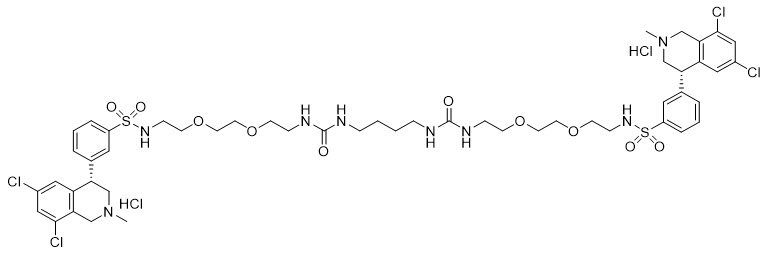
11 DESCRIPTIONIBSRELA (tenapanor) tablets contain tenapanor hydrochloride as an active ingredient. Tenapanor hydrochloride is a sodium/hydrogen exchanger 3 (NHE3) inhibitor for oral use. The chemical name for ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tenapanor is a locally acting inhibitor of the sodium/hydrogen exchanger 3 (NHE3), an antiporter expressed on the apical surface of the small intestine and colon ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of tenapanor was assessed in a 6-month carcinogenicity study in Tg rasH2 mice and in a ...
-
14 CLINICAL STUDIESThe efficacy of IBSRELA for the treatment of IBS-C was established in two double-blind, placebo-controlled, randomized, multicenter trials in adult patients: Trial 1 (TEN-01-302; NCT02686138) and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIBSRELA tablets contain 50 mg tenapanor and are oval, white to off-white, debossed with "50" on one side and "5791" on the other side. IBSRELA is supplied in a white, opaque, high-density ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patients to read the FDA-approved patient labeling (Medication Guide). Diarrhea - Instruct patients to stop IBSRELA and contact their healthcare provider if they experience severe ...
-
SPL UNCLASSIFIED SECTIONManufactured for and distributed by Ardelyx, Inc. Waltham, MA 02451 USA - IBSRELA® is a registered trademark of Ardelyx, Inc. Patent: www.IBSRELA-patents.com
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: May 2021 - Medication Guide - IBSRELA® (ibs rel`a) (tenapanor) tablets, for oral use - What is the ...
-
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label - 050-60NDC 73154-050-60 - IBSRELA® (tenapanor) tablets - 50 mg - ATTENTION PHARMACIST: Dispense the accompanying - Medication Guide to each patient. Attention Pharmacist: Dispense IBSRELA® in original - container ...
-
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label - 050-06PROFESSIONAL SAMPLE – NOT FOR SALE - NDC 73154-050-06 - IBSRELA® (tenapanor) tablets - 50 mg - ATTENTION PHARMACIST: Dispense the - accompanying Medication Guide to each patient. Attention ...
-
INGREDIENTS AND APPEARANCEProduct Information



 50" on one side and "5791" on the other side.
50" on one side and "5791" on the other side.