Label: HUMATROPE- somatropin kit
-
NDC Code(s):
0002-7554-01,
0002-7555-01,
0002-7556-01,
0002-7618-01, view more0002-7619-01, 0002-8147-01, 0002-8148-01, 0002-8149-01
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HUMATROPE safely and effectively. See full prescribing information for HUMATROPE. HUMATROPE® (somatropin) for injection, for ...These highlights do not include all the information needed to use HUMATROPE safely and effectively. See full prescribing information for HUMATROPE.
HUMATROPE® (somatropin) for injection, for subcutaneous use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
HUMATROPE is a recombinant human growth hormone indicated for:
- Pediatric Patients: growth failure due to inadequate secretion of endogenous growth hormone (GH); short stature associated with Turner syndrome; Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range; short stature or growth failure in short stature homeobox-containing gene (SHOX) deficiency; short stature born small for gestational age (SGA) with no catch-up growth by 2 years to 4 years of age. (1.1)
- Adult Patients: replacement of endogenous GH in adults with GH deficiency. (1.2)
DOSAGE AND ADMINISTRATION
- Administer by subcutaneous injection to the back of upper arm, abdomen, buttock, or thigh with regular rotation of injection sites. (2.1)
- Pediatric Dosage - divide the calculated weekly dosage into equal doses given either 6, or 7 days per week.
-
Adult Dosage - Either of the following two dosing regimens may be used:
- Non-weight based dosing: Initiate with a dose of approximately 0.2 mg/day (range: 0.15 mg/day-0.3 mg/day) and increase the dose every 1-2 months by increments of approximately 0.1 mg/day-0.2 mg/day, according to individual patient requirements (2.3)
- Weight-based dosing (Not recommended for obese patients): Initiate at 0.006 mg/kg daily and increase the dose according to individual patient requirements to a maximum of 0.0125 mg/kg daily (2.3)
- See Full Prescribing Information for reconstitution instructions. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection:
- 6 mg, 12 mg, or 24 mg lyophilized powder in a single-patient-use cartridge for reconstitution. (3)
CONTRAINDICATIONS
- Acute critical illness. (4)
- Pediatric patients with Prader-Willi syndrome who are severely obese, have history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to risk of sudden death. (4)
- Active malignancy. (4)
- Hypersensitivity to somatropin or excipients. (4)
- Active proliferative or severe non-proliferative diabetic retinopathy. (4)
- Pediatric patients with closed epiphyses. (4)
WARNINGS AND PRECAUTIONS
- Increased Risk of Neoplasm: Second neoplasms have occurred in childhood cancer survivors. Monitor patients with preexisting tumors for progression or recurrence. (5.3)
- Glucose Intolerance and Diabetes Mellitus: HUMATROPE may decrease insulin sensitivity, particularly at higher doses. Monitor glucose levels periodically in all patients receiving HUMATROPE, especially in patients with existing diabetes mellitus or at risk for development. (5.4)
- Intracranial Hypertension (IH): Has been reported usually within 8 weeks of initiation. Perform fundoscopic examinations prior to initiation and periodically thereafter. If papilledema occurs, stop treatment. (5.5)
- Hypersensitivity: Serious hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention. (5.6)
- Fluid Retention: May occur in adults and may be dose dependent. (5.7)
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism. (5.8)
- Hypothyroidism: Monitor thyroid function periodically as hypothyroidism may occur or worsen after initiation of somatropin. (5.9)
- Slipped Capital Femoral Epiphysis in Pediatric Patients: May occur; evaluate patients with onset of a limp or hip/knee pain. (5.10)
- Progression of Preexisting Scoliosis in Pediatric Patients: Monitor patients with scoliosis for progression. (5.11)
- Pancreatitis: Has been reported; consider pancreatitis in patients with abdominal pain, especially pediatric patients. (5.12)
ADVERSE REACTIONS
Common adverse reactions reported in adult and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, carpal tunnel syndrome, peripheral edema, flu syndrome, hypothyroidism, hyperglycemia, and impaired glucose tolerance. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Replacement Glucocorticoid Treatment: Patients treated with glucocorticoids for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of HUMATROPE. (7)
- Non-Replacement Glucocorticoid Treatment in Pediatric Patients: Adjust glucocorticoid dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth. (7)
- Cytochrome P450-Metabolized Drugs: HUMATROPE may alter the clearance. Monitor carefully if used with HUMATROPE. (7)
- Oral Estrogen: Patients may require larger doses of HUMATROPE. (7)
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required. (5.4, 7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
1.2 Adult Patients
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Use Instructions
2.2 Pediatric Dosage
2.3 Adult Dosage
2.4 Reconstitution of Cartridges
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness
5.2 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
5.3 Increased Risk of Neoplasms
5.4 Glucose Intolerance and Diabetes Mellitus
5.5 Intracranial Hypertension
5.6 Severe Hypersensitivity
5.7 Fluid Retention
5.8 Hypoadrenalism
5.9 Hypothyroidism
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
5.12 Pancreatitis
5.13 Lipoatrophy
5.14 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pediatric Patients with Growth Failure Due to Inadequate Secretion of Endogenous Growth Hormone
14.2 Pediatric Patients with Short Stature Associated with Turner Syndrome
14.3 Pediatric Patients with Idiopathic Short Stature (ISS)
14.4 Pediatric Patients with Short Stature or Growth Failure in SHOX Deficiency
14.5 Pediatric Patients with Short Stature Born Small for Gestational Age (SGA) Who Fail to Demonstrate Catch-up Growth by Age 2 - 4 Years
14.6 Adult Patients with Growth Hormone Deficiency
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pediatric Patients - HUMATROPE is indicated for the treatment of pediatric patients with: growth failure due to inadequate secretion of endogenous growth hormone (GH), short stature ...
1.1 Pediatric Patients
HUMATROPE is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH),
- short stature associated with Turner syndrome,
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range,
- short stature or growth failure in short stature homeobox-containing gene (SHOX) deficiency,
- short stature born small for gestational age (SGA) with no catch-up growth by 2 years to 4 years of age.
Close1.2 Adult Patients
HUMATROPE is indicated for the replacement of endogenous GH in adults with GH deficiency.
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Use Instructions - Therapy with HUMATROPE should be supervised by a physician who is experienced in the diagnosis and management of patients with the conditions for which ...
2.1 Administration and Use Instructions
- Therapy with HUMATROPE should be supervised by a physician who is experienced in the diagnosis and management of patients with the conditions for which HUMATROPE is indicated [see Indications and Usage (1)].
- Fundoscopic examination should be performed routinely before initiating treatment with HUMATROPE to exclude preexisting papilledema, and periodically thereafter [see Warnings and Precautions (5.5)].
- Leave HUMATROPE at room temperature for 10 minutes prior to administration.
- Administer HUMATROPE by subcutaneous injection to the back of the upper arm, abdomen, buttock, or thigh with regular rotation of injection sites to avoid lipoatrophy.
2.2 Pediatric Dosage
- Individualize dosage for each patient based on the growth response.
- Divide the calculated weekly HUMATROPE dosage into equal doses given either 6 or 7 days per week.
- The recommended weekly dose in milligrams (mg) per kilogram (kg) of body weight for pediatric patients is:
- Pediatric GH Deficiency: 0.18 mg/kg/week to 0.3 mg/kg/week (0.026 to 0.043 mg/kg/day)
- Turner Syndrome: Up to 0.375 mg/kg/week (up to.054 mg/kg/day)
- Idiopathic Short Stature: Up to 0.37 mg/kg/week (up to 0.053 mg/kg/day)
- SHOX Deficiency: 0.35 mg/kg/week (0.05 mg/kg/day)
-
Small for Gestational Age (SGA): Up to 0.47 mg/kg/week (up to 0.067 mg/kg/day)
- In very short pediatric patients, height SDS less than -3, and older pubertal pediatric patients consider initiating treatment with a larger dose of HUMATROPE (up to 0.067 mg/kg/day). Consider a gradual reduction in dosage if substantial catch-up growth is observed during the first few years of therapy. In pediatric patients less than 4 years of age with less severe short stature, baseline height SDS values between -2 and -3, consider initiating treatment at 0.033 mg/kg/day and titrate the dose as needed.
- Assess compliance and evaluate other causes of poor growth such as hypothyroidism, under-nutrition, advanced bone age and antibodies to recombinant human GH if patients experience failure to increase height velocity, particularly during the first year of treatment.
- Discontinue HUMATROPE for stimulation of linear growth once epiphyseal fusion has occurred [see Contraindications (4)].
2.3 Adult Dosage
- Patients who were treated with somatropin for GH deficiency in childhood and whose epiphyses are closed should be reevaluated before continuation of somatropin for GH deficient adults.
- Consider using a lower starting dose and smaller dose increment increases for geriatric patients as they may be at increased risk for adverse reactions with HUMATROPE than younger individuals [see Use in Specific Populations (8.5)].
- Women may require higher doses and patients receiving oral estrogen may require higher doses [see Drug Interactions (7)].
- Administer the prescribed dose daily.
- Either of two HUMATROPE dosing regimens may be used:
- Non-weight based:
- Initiate HUMATROPE with a dose of approximately 0.2 mg/day (range, 0.15 mg/day to 0.3 mg/day) and increase the dose every 1-2 months by increments of approximately 0.1 mg/day to 0.2 mg/day, according to individual patient requirements based on the clinical response and serum insulin-like growth factor 1 (IGF-1) concentrations.
- Use the patient's clinical response, adverse reactions, and determination of age- and gender-adjusted serum IGF-1 concentrations as guidance in dose titration.
- Maintenance dosages will vary considerably from person to person, and between male and female patients.
- Weight-based:
- Initiate HUMATROPE at 0.006 mg/kg daily and increase the dose according to individual patient requirements to a maximum of 0.0125 mg/kg daily.
- Use the patient's clinical response, adverse reactions, and determination of age- and gender-adjusted serum IGF-1 concentrations as guidance in dose titration.
- Maintenance dosages will vary considerably from person to person, and between male and female patients
- Not recommended for obese patients as they are more likely to experience adverse reactions with this regimen.
- Non-weight based:
Close2.4 Reconstitution of Cartridges
- Each single-patient-use HUMATROPE cartridge is designed for use only with the appropriate corresponding HumatroPen®.
- Reconstitute each cartridge of HUMATROPE using only the diluent syringe that accompanies the cartridge. Do not shake. The reconstituted solution should be clear.
- Inspect visually for particulate matter and discoloration. If the resulting solution is cloudy or contains particulate matter do not use.
- The somatropin concentrations for the reconstituted HUMATROPE cartridges are as follows in Table 1:
Table 1: Somatropin Concentration of HUMATROPE Cartridges. Cartridge Somatropin Concentration 6 mg 2.08 mg/mL 12 mg 4.17 mg/mL 24 mg 8.33 mg/mL
- Reconstituted cartridges of HUMATROPE are stable for up to 28 days when refrigerated at 36° to 46°F (2° to 8°C). Do not leave at room temperature more than 30 minutes per day. Avoid freezing the reconstituted cartridge. Protect HUMATROPE from light during storage.
- For patients with known hypersensitivity to the diluent supplied with the HUMATROPE cartridge [see Warnings and Precautions (5.6)], do not use HUMATROPE cartridge.
-
3 DOSAGE FORMS AND STRENGTHS
HUMATROPE is a white lyophilized powder available as follows: For injection: 6 mg in a single-patient-use cartridge (gold) For injection: 12 mg in a single-patient-use cartridge (teal) For ...
HUMATROPE is a white lyophilized powder available as follows:
- For injection: 6 mg in a single-patient-use cartridge (gold)
- For injection: 12 mg in a single-patient-use cartridge (teal)
- For injection: 24 mg in a single-patient-use cartridge (purple)
-
4 CONTRAINDICATIONS
HUMATROPE is contraindicated in patients with: Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure ...
HUMATROPE is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin [see Warnings and Precautions (5.1)].
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death [see Warnings and Precautions (5.2)].
- Active malignancy [see Warnings and Precautions (5.3)].
- Known hypersensitivity to somatropin or any of the excipients in HUMATROPE. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins [see Warnings and Precautions (5.6)].
- Active proliferative or severe non-proliferative diabetic retinopathy.
- Pediatric patients with closed epiphyses.
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness - Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or ...
5.1 Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic doses of somatropin [see Contraindications (4)]. Two placebo-controlled clinical studies in non-GH deficient adult patients (n=522) with these conditions in intensive care units revealed a significant increase in mortality (42% vs. 19%) among somatropin-treated patients (doses 5.3-8.0 mg/day) compared to those receiving placebo. The safety of continuing HUMATROPE treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. HUMATROPE is not indicated for the treatment of non-GH deficient adults.
5.2 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
There have been reports of sudden death after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. If, during treatment with somatropin, patients show signs of upper airway obstruction (including onset of, or increased, snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with Prader-Willi syndrome treated with somatropin should also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively [see Contraindications (4)]. HUMATROPE is not indicated for the treatment of pediatric patients who have growth failure due to Prader-Willi syndrome.
5.3 Increased Risk of Neoplasms
Active Malignancy
There is an increased risk of malignancy progression with somatropin treatment in patients with active malignancy [see Contraindications (4)]. Any preexisting malignancy should be inactive and its treatment complete prior to instituting therapy with HUMATROPE. Discontinue HUMATROPE if there is evidence of recurrent activity.
Risk of Second Neoplasm in Pediatric Patients
An increased risk of a second neoplasm in pediatric cancer survivors who were treated with radiation to the brain/head and who developed subsequent GH deficiency and were treated with somatropin has been reported. Intracranial tumors, in particular meningiomas, were the most common of these second neoplasms. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence. Monitor all patients receiving HUMATROPE who have a history of GH deficiency secondary to an intracranial neoplasm for progression or recurrence of the tumor.
New Malignancy During Treatment
Because pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies, thoroughly consider the risks and benefits of starting HUMATROPE in these patients. If HUMATROPE is initiated, carefully monitor patients for development of neoplasms.
Monitor all patients receiving HUMATROPE carefully for increased growth, or potential malignant changes, of preexisting nevi. Advise patients/caregivers to report marked changes in behavior, onset of headaches, vision disturbances and/or changes in skin pigmentation or changes in the appearance of pre-existing nevi.
5.4 Glucose Intolerance and Diabetes Mellitus
Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New onset type 2 diabetes mellitus has been reported in patients taking somatropin. Previously undiagnosed impaired glucose tolerance and overt diabetes mellitus may be unmasked. Monitor glucose levels periodically in all patients receiving HUMATROPE, especially in those with risk factors for diabetes mellitus, such as obesity, Turner syndrome, or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or impaired glucose tolerance should be monitored closely. The doses of antidiabetic agents may require adjustment when HUMATROPE is initiated.
5.5 Intracranial Hypertension
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in a small number of patients treated with somatropins. Symptoms usually occurred within the first eight (8) weeks after the initiation of somatropin therapy. In all reported cases, IH-associated signs and symptoms rapidly resolved after cessation of therapy or a reduction of the somatropin dose. Fundoscopic examination should be performed routinely before initiating treatment with HUMATROPE to exclude preexisting papilledema, and periodically thereafter. If papilledema is observed by fundoscopy during somatropin treatment, treatment should be stopped. If somatropin-induced IH is diagnosed, treatment with HUMATROPE can be restarted at a lower dose after IH-associated signs and symptoms have resolved. Patients with Turner syndrome may be at increased risk for the development of IH.
5.6 Severe Hypersensitivity
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins. Patients and caregivers should be informed that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs. Do not use HUMATROPE in patients with known hypersensitivity to somatropin or any of the excipients in HUMATROPE. Do not use HUMATROPE cartridges in patients with known hypersensitivity to metacresol or glycerin [see Dosage and Administration (2.4), Contraindications (4)].
5.7 Fluid Retention
Fluid retention during somatropin replacement therapy may frequently occur. Clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paresthesia) are usually transient and dose dependent.
5.8 Hypoadrenalism
Patients receiving somatropin therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of HUMATROPE treatment. Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism [see Drug Interactions (7)].
5.9 Hypothyroidism
Undiagnosed/untreated hypothyroidism may prevent an optimal response to HUMATROPE, in particular, the growth response in pediatric patients. Patients with Turner syndrome have an inherently increased risk of developing autoimmune thyroid disease and primary hypothyroidism. In patients with GH deficiency, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Therefore, patients treated with somatropin should have periodic thyroid function tests performed, and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
Slipped capital femoral epiphysis may occur more frequently in patients undergoing rapid growth. Evaluate pediatric patients with the onset of a limp or complaints of hip or knee pain.
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
Somatropin increases the growth rate, and progression of existing scoliosis can occur in patients who experience rapid growth. Somatropin has not been shown to increase the occurrence of scoliosis. Monitor patients with a history of scoliosis for progression of scoliosis.
5.12 Pancreatitis
Cases of pancreatitis have been reported in pediatric patients and adults receiving somatropins. The risk may be greater risk in pediatric patients compared with adults. Published literature indicates that girls who have Turner syndrome may be at greater risk than other pediatric patients receiving somatropins. Pancreatitis should be considered in patients who develop abdominal pain.
5.13 Lipoatrophy
When somatropins are administered subcutaneously at the same site over a long period of time, tissue atrophy may result. Rotate injection sites when administering HUMATROPE to reduce this risk [see Dosage and Administration (2.1)].
Close5.14 Laboratory Tests
Serum levels of inorganic phosphorus, alkaline phosphatase, parathyroid hormone and IGF-I may increase after HUMATROPE therapy.
-
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling: Increased mortality in patients with acute critical illness [see Warnings and Precautions ...
The following important adverse reactions are also described elsewhere in the labeling:
- Increased mortality in patients with acute critical illness [see Warnings and Precautions (5.1)]
- Fatalities in children with Prader-Willi syndrome [see Warnings and Precautions (5.2)]
- Neoplasms [see Warnings and Precautions (5.3)]
- Glucose intolerance and diabetes mellitus [see Warnings and Precautions (5.4)]
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- Severe hypersensitivity [see Warnings and Precautions (5.6)]
- Fluid retention [see Warnings and Precautions (5.7)]
- Hypoadrenalism [see Warnings and Precautions (5.8)]
- Hypothyroidism [see Warnings and Precautions (5.9)]
- Slipped capital femoral epiphysis in pediatric patients [see Warnings and Precautions (5.10)]
- Progression of preexisting scoliosis in pediatric patients [see Warnings and Precautions (5.11)]
- Pancreatitis [see Warnings and Precautions (5.12)]
- Lipoatrophy [see Warnings and Precautions (5.13)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under varying conditions, adverse reaction rates observed during the clinical studies performed with one somatropin formulation cannot always be directly compared to the rates observed during the clinical studies performed with a different somatropin formulation, and may not reflect the adverse reaction rates observed in practice.
Pediatric Patients
Growth Failure Due to Inadequate Secretion of Endogenous Growth Hormone
In an uncontrolled open-label study, 314 treatment-naive children aged >2 years who had GH deficiency were treated with HUMATROPE (0.06 mg/kg 3 times per week) for up to 8 years. Adverse reactions of special interest are reported in Table 2.
Table 2: Adverse Reactions of Special Interest Occurring in Humatrope-Treated Patients with Growth Failure Due to Inadequate Secretion of Endogenous Growth Hormone in an Open-label Study for Up to 8 Years a Dose=0.06 mg/kg 3 times per week for up to 8 years.
b n=1
Adverse Reaction HUMATROPEa
(n=314)Hypothyroidism 25% Allergic reaction 11% Arthralgia 6% Bone disorder 4% Edema 4% Injection site pain/reaction 4% Neoplasm/tumor 2% Cardiovascular disorders 1% Thyroid disorders 1% Intracranial hypertension 0%b Short Stature Associated with Turner Syndrome
In a randomized, concurrent-controlled (untreated), open-label study until attainment of adult height, the adverse reactions of special interest occurring in 74 patients treated with Humatrope at dose 0.3 mg/kg/week (mean duration 4.1 years) and in 62 untreated patients (mean duration 3.7 years) are reported in Table 3. A similar increase in otitis media was observed in an 18-month placebo-controlled study.
Table 3: Adverse Reactions of Special Interest Occurring in Patients with Turner Syndrome in an Open-label Study Until Attainment of Adult Height Untreated
(n=62)HUMATROPE
(n=74)Surgical procedure 27% 45% Otitis media 26% 43% Ear disorders 5% 18% Idiopathic Short Stature
Adverse reactions occurring in a randomized, placebo-controlled study of HUMATROPE treatment (0.22 mg/kg/week) until attainment of adult height (mean duration of HUMATROPE treatment 3.7 years, mean duration of placebo treatment 3.3 years) are reported in Table 4. Mean fasting serum insulin concentration increased by 10% in the HUMATROPE treatment group at the end of treatment relative to baseline, but remained within the normal reference range.
Table 4: Adverse Reactions Occurring in Patients with Idiopathic Short Stature Treated with HUMATROPE in a Randomized Placebo-controlled Study Placebo
(n=31)HUMATROPE
(n=37)Scoliosis 13% 19% Otitis media 7% 16% Hyperlipidemia 3% 8% Gynecomastia 3% 5% Hip pain 0 3% Arthralgia 3% 11% Arthrosis 7% 11% Myalgia 13% 24% Hypertension 0 3% In a dose-response study (239 patients treated for 2 years), among HUMATROPE dose groups [0.24 mg/kg/week (n=78), 0.37 mg/kg/week (n=83), 0.24 mg/kg/week for the first year and 0.37 mg/kg/week thereafter (n=78)], mean fasting blood glucose, mean glycosylated hemoglobin, and the incidence of elevated fasting blood glucose concentrations were similar. One patient developed glucose intolerance and high serum HbA1c.
Short Stature or Growth Failure in SHOX Deficiency
Adverse reactions of special interest from a 2-year randomized, open-label study with HUMATROPE (0.35 mg/kg/wk) compared to no treatment are presented in Table 5. During the 2-year study period, the proportion of patients who had at least one IGF-I concentration greater than 2.0 SD above the age- and gender-appropriate mean was 10 of 27 (37%) for the HUMATROPE-treated group vs. 0 of 24 patients (0%) for the untreated group. The proportion of patients who had at least one IGFBP-3 concentration greater than 2.0 SD above the age and gender appropriate mean was 16 of 27 (59%) for the HUMATROPE treated group vs. 7 of 24 (29%) for the untreated group.
Table 5: Adverse Reactions of Special Interest Occurring in Patients with SHOX Deficiency By Treatment Group in an Open-label Study a Percentage calculated for males only: Untreated (0/1), HUMATROPE (1/12)
Untreated
(n=25)HUMATROPE
(n=27)Arthralgia 8% 11% Gynecomastiaa 0% 8% Excessive number of cutaneous nevi 0% 7% Scoliosis 0% 47% Small for Gestational Age (SGA) with No Catch-up Growth by Age 2-4 Years
In a 2-year, multicenter, randomized study, 193 pediatric patients were treated with 2 different HUMATROPE treatment regimens: a fixed dose of 0.067 mg/kg/day (FHD group) or an individually adjusted dose regimen (IAD group; starting dose 0.035 mg/kg/day which could be increased as early as Month 3 to 0.067 mg/kg/day based on a validated growth prediction model). Reported adverse reactions included: common childhood infectious diseases, otitis media, headaches, and slipped capital femoral epiphysis (n=1. Six patients (4 in the FHD group and 2 in the IAD group whose dose was increased from 0.035 mg/kg/day to 0.067 mg/kg/day [one at Month 3 and one at Year 1]) had impaired fasting glucose at Year 2. Two of 6 had impaired fasting glucose during the study, and one discontinued HUMATROPE at month 15 as a consequence. At study completion, 20-25% of patients had serum IGF-I SDS values > +2.
The following adverse reactions were reported from an observational study of 340 pediatric patients who received HUMATROPE with an average dosage of 0.041 mg/kg/day (maximum dose: 0.084 mg/kg/day) for an average of 3.0 years: type 2 diabetes mellitus (n=1), carpal tunnel syndrome (n=1) and an exacerbation of preexisting scoliosis (n=1).
Adult Patients with GH deficiency
Adult-Onset GH Deficiency
In the first 6 months of controlled blinded studies during which patients received either HUMATROPE or placebo, patients who received HUMATROPE experienced an increase in edema (17% vs. 4%) and peripheral edema (12% vs. 0%). Edema, muscle pain, joint pain, and joint disorder were reported early in therapy and tended to be transient or responsive to dosage titration.
Two of 113 patients developed carpal tunnel syndrome after beginning maintenance therapy without a low dose (0.00625 mg/kg/day) lead-in phase. Symptoms abated in these patients after dosage reduction.
Adverse reactions with ≥5% overall occurrence rate during 12 or 18 months of replacement therapy with HUMATROPE are shown in Table 6 (adult-onset patients) and in Table 7 (childhood-onset patients).
Table 6: Adverse Reactions with ≥5% Overall Occurrence in Adult-Onset Growth Hormone-Deficient Patients Treated with HUMATROPE for 18 Months as Compared with 6-Month Placebo and 12-Month HUMATROPE Exposure Adverse Reaction 18 Months Exposure
[Placebo (6 Months)/Humatrope (12 Months)]
(n=44)18 Months Humatrope Exposure
(n=52)Edema 11% 21% Arthralgia 14% 17% Paresthesia 14% 17% Myalgia 9% 14% Pain 14% 14% Peripheral edema 18% 12% Headache 7% 8% Hypertension 5% 8% Joint disorder 2% 6% Rhinitis 11% 13% Back pain 9% 10% Acne 0% 6% Surgical procedure 2% 6% Flu syndrome 7% 4% Childhood-Onset GH Deficiency
Two double-blind, placebo-controlled studies were conducted in 67 adult patients who had received previous somatropin treatment during childhood. Patients were randomized to receive either placebo injections or HUMATROPE (0.00625 mg/kg/day for the first 4 weeks, then 0.0125 mg/kg/day thereafter) for the first 6 months, followed by open-label use of HUMATROPE for the next 12 months for all patients. During the placebo-controlled phase (first 6 months) of the study, elevations of serum glutamic oxaloacetic transferase were reported more for HUMATROPE-treated (13% vs. 0%) than placebo-treated patients.
Table 7: Adverse Reactions with ≥5% Overall Occurrence in Childhood-Onset Growth Hormone-Deficient Patients Treated with HUMATROPE for 18 Months as Compared with 6-Month Placebo and 12-Month HUMATROPE Exposure a Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase
Adverse Reaction 18 Months Exposure
[Placebo (6 Months)/Humatrope (12 Months)]
(n=30)18 Months Humatrope Exposure
(n=32)AST a increased 7% 13% Headache 7% 9% Asthenia 3% 6% Edema 10% 6% Myalgia 7% 6% Pain 10% 6% ALT a increased 7% 6% Flu syndrome 10% 16% Cough increased 0% 6% Hypesthesia 0% 6% Rhinitis 7% 6% Respiratory disorder 7% 3% Gastritis 7% 0% Pharyngitis 7% 3% Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of HUMATROPE. Because these adverse events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Severe Hypersensitivity Reactions — Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins
Neurologic — Headaches (common in pediatric patients and occasional in adults)
Skin — Increase in size or number of cutaneous nevi
Endocrine — Gynecomastia
Gastrointestinal — Pancreatitis
Metabolic — New-onset type 2 diabetes mellitus
Neoplasia — Leukemia has been reported in a small number of GH deficient pediatric patients treated with somatropin
-
7 DRUG INTERACTIONS
Table 8 includes a list of drugs with clinically important drug interactions when administered concomitantly with HUMATROPE and instructions for preventing or managing them. Table 8 ...
Table 8 includes a list of drugs with clinically important drug interactions when administered concomitantly with HUMATROPE and instructions for preventing or managing them.
CloseTable 8: Clinically Important Drug Interactions with HUMATROPE Replacement Glucocorticoid Treatment Clinical Impact: Microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. HUMATROPE inhibits 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Initiation of HUMATROPE may result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations. Intervention: Patients treated with glucocorticoid replacement for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of HUMATROPE [see Warnings and Precautions (5.8)]. Examples: Cortisone acetate and prednisone may be effected more than others since conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1. Non-Replacement Glucocorticoid Treatment in Pediatric Patients Clinical Impact: Non-replacement glucocorticoid treatment, including supraphysiologic glucocorticoid treatment, may attenuate the growth promoting effects of HUMATROPE in pediatric patients. Intervention: Carefully adjust glucocorticoid dosing in pediatric patients receiving glucocorticoid treatments to avoid both hypoadrenalism and an inhibitory effect on growth. Cytochrome P450-Metabolized Drugs Clinical Impact: Limited published data indicate that somatropin treatment increases cytochrome P450 (CP450)-mediated antipyrine clearance. HUMATROPE may alter the clearance of compounds known to be metabolized by CP450 liver enzymes. Intervention: Careful monitoring is advisable when HUMATROPE is administered in combination with drugs metabolized by CP450 liver enzymes. Oral Estrogen Clinical Impact: Oral estrogens may reduce the serum IGF-1 response to HUMATROPE. Intervention: Patients receiving oral estrogen may require greater HUMATROPE dosages [see Dosage and Administration (2.3)]. Insulin and/or Other Hypoglycemic Agents Clinical Impact: Treatment with HUMATROPE may decrease insulin sensitivity, particularly at higher doses. Intervention: Patients with diabetes mellitus may require adjustment of their doses of insulin and/or other hypoglycemic agents [see Warnings and Precautions (5.4)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Limited available data with somatropin use in pregnant women are insufficient to determine a drug-associated risk of adverse developmental outcomes. Animal ...
8.1 Pregnancy
Risk Summary
Limited available data with somatropin use in pregnant women are insufficient to determine a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have not been conducted with HUMATROPE.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of somatropin in human milk. Limited published data indicate that exogenous somatropin does not increase normal breastmilk concentrations of growth hormone. No adverse effects related to somatropin in the breastfed infant have been reported. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HUMATROPE and any potential adverse effects on the breastfed infant from HUMATROPE or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of HUMATROPE in pediatric patients have been established in growth failure due to inadequate secretion of endogenous growth hormone, short stature associated with Turner syndrome, idiopathic short stature (ISS), short stature or growth failure in SHOX deficiency, and short stature in children born small for gestational age (SGA) with no catch-up growth by 2 years to 4 years of age.
Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
Safety and effectiveness of HUMATROPE have been established in pediatric patients with growth failure due to growth hormone deficiency based on data from an open-label, uncontrolled, multicenter study with HUMATROPE in 314 pediatric patients conducted for up to 8 years [see Clinical Studies (14.1)].
Short Stature Associated with Turner Syndrome
Safety and effectiveness of HUMATROPE have been established in pediatric patients with short stature associated with Turner syndrome based on data from one long-term, randomized, open-label, multicenter, concurrently controlled study; two long-term, open-label multicenter, historically controlled US studies; and one long-term, randomized, US dose-response study with HUMATROPE in 181 pediatric patients [see Clinical Studies (14.2)].
Idiopathic Short Stature (ISS)
Safety and effectiveness of HUMATROPE have been established in pediatric patients with ISS based on data from two randomized, multicenter studies, one placebo-controlled study and one dose-response study with HUMATROPE in 310 pediatric patients [see Clinical Studies (14.3)].
Short Stature or Growth Failure in SHOX Deficiency
Safety and effectiveness of HUMATROPE have been established in pediatric patients with short stature or growth failure in SHOX deficiency based on data from a randomized, controlled, two-year, three-arm, open-label study with HUMATROPE in 52 pediatric patients [see Clinical Studies (14.4)].
Short Stature in Children Born Small for Gestational Age (SGA) with No Catch-up Growth by 2 Years to 4 Years of Age
Safety and effectiveness of HUMATROPE have been established in pediatric patients with short stature born SGA with no catch-up growth based on data from two clinical studies with HUMATROPE in 214 pediatric patients [see Clinical Studies (14.5)].
Close8.5 Geriatric Use
The safety and effectiveness of HUMATROPE in patients aged 65 years and over has not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of somatropin, and therefore may be more prone to development of adverse reactions. A lower starting dose and smaller dose increments should be considered for older patients [see Dosage and Administration (2.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - HUMATROPE contains somatropin, which is not a controlled substance. 9.2 Abuse - Inappropriate use of somatropin may result in significant negative health ...
-
10 OVERDOSAGE
Acute overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Overdose with somatropin is likely to cause fluid retention. Long-term overdosage could result in signs and ...
Acute overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Overdose with somatropin is likely to cause fluid retention. Long-term overdosage could result in signs and symptoms of gigantism and/or acromegaly consistent with the known effects of excess endogenous growth hormone.
Close -
11 DESCRIPTION
Somatropin is a human growth hormone (GH) produced by recombinant DNA technology using Escherichia coli. The protein is comprised of 191 amino acid residues and has a molecular weight of about ...
Somatropin is a human growth hormone (GH) produced by recombinant DNA technology using Escherichia coli. The protein is comprised of 191 amino acid residues and has a molecular weight of about 22,125 daltons. The amino acid sequence is identical to that of human GH of pituitary origin.
HUMATROPE (somatropin) for injection is a sterile, white, lyophilized powder intended for subcutaneous injection after reconstitution supplied in a cartridge. Phosphoric acid and/or sodium hydroxide may have been added to adjust the pH. Reconstituted solutions have a pH of approximately 7.5. This product is oxygen sensitive.
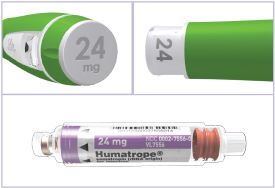
Cartridge — Each single-patient-use cartridge of HUMATROPE contains either 6 mg (18 IU), 12 mg (36 IU), or 24 mg (72 IU) of somatropin. Each HUMATROPE cartridge contains the following components (see Table 9):
Table 9: Contents of HUMATROPE Component Cartridge 6 mg
(gold)12 mg
(teal)24 mg
(purple)Somatropin 6 mg 12 mg 24 mg Dibasic sodium phosphate 1.36 mg 2.72 mg 5.43 mg Glycine 6 mg 12 mg 24 mg Mannitol 18 mg 36 mg 72 mg Each cartridge is co-packaged with an accompanying syringe containing approximately 3 mL of diluent containing Water for Injection with 0.3% metacresol as a preservative and 1.7%, 0.29%, and 0.29% glycerin in the 6, 12, and 24 mg cartridges, respectively.
Close -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Somatropin binds to dimeric GH receptors located within the cell membranes of target tissue cells. This interaction results in intracellular signal transduction and ...
12.1 Mechanism of Action
Somatropin binds to dimeric GH receptors located within the cell membranes of target tissue cells. This interaction results in intracellular signal transduction and subsequent induction of transcription and translation of GH-dependent proteins including IGF-1, IGF BP-3 and acid-labile subunit. Somatropin has direct tissue and metabolic effects or mediated indirectly by IGF-1, including stimulation of chondrocyte differentiation, and proliferation, stimulation of hepatic glucose output, protein synthesis and lipolysis.
Somatropin stimulates skeletal growth in pediatric patients with GHD as a result of effects on the growth plates (epiphyses) of long bones. The stimulation of skeletal growth increases linear growth rate (height velocity) in most somatropin-treated pediatric patients. Linear growth is facilitated in part by increased cellular protein synthesis.
12.2 Pharmacodynamics
Subcutaneous administration of a single dose of Humatrope (0.033 mg/kg body weight) in healthy volunteers (10 males, 10 females) resulted in an increased median IGF-1 level from 202 ng/mL (men) and 107 ng/mL (women) predose to maximal level of 362 ng/mL (men) and 234 ng/mL (women) after a median of 21 hours (men) and 14 hours (women).
12.3 Pharmacokinetics
Absorption
HUMATROPE has been studied following intramuscular, subcutaneous, and intravenous administration in healthy adult subjects. A single subcutaneous dose administration of HUMATROPE 0.1 mg/kg (0.27 IU/kg) in healthy subjects (n= 8) resulted in a mean (SD) Cmax of 63.3 (18.2) ng/mL at median Tmax of 4.0 (range 3 to 8) hours. The absolute bioavailability of somatropin is 75% after subcutaneous administration.
Distribution
The mean (SD) volume of distribution of somatropin after single dose subcutaneous injection of 0.1 mg/kg (0.27 IU/kg) in healthy subjects is about 0.96 (0.3) L/kg.
Elimination
Metabolism — Extensive metabolism studies have not been conducted. The metabolic fate of somatropin involves classical protein catabolism in both the liver and kidneys. In renal cells, at least a portion of the breakdown products of somatropin is returned to the systemic circulation.
Excretion — In healthy subjects, mean somatropin clearance is 0.18 (0.03) L/hr/kg following subcutaneous administration. The mean half-life of subcutaneous somatropin is 3.8 (1.4) hours. The long half-life observed after subcutaneous administration is due to slow absorption from the injection site. Urinary excretion of intact HUMATROPE has not been measured. Small amounts of somatropin have been detected in the urine of pediatric patients following replacement therapy.
Specific Populations
Geriatric patients — The pharmacokinetics of HUMATROPE have not been studied in patients greater than 65 years of age.
Pediatric patients — The pharmacokinetics of HUMATROPE in pediatric patients are similar to those of adults.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of HUMATROPE or other somatropins.
In a clinical study with HUMATROPE during the first 6 months of HUMATROPE therapy in 314 naive patients, 1.6% developed specific antibodies to HUMATROPE (binding capacity ≥0.02 mg/L). None had antibody concentrations which exceeded 2 mg/L. Throughout 8 years of this same study, two patients (0.6%) had binding capacity >2 mg/L. Neither patient demonstrated a decrease in growth velocity at or near the time of increased antibody production. It has been reported that growth attenuation from pituitary-derived GH may occur when antibody concentrations are >1.5 mg/L.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity and impairment of fertility studies have not been conducted with HUMATROPE.Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity and impairment of fertility studies have not been conducted with HUMATROPE.
-
14 CLINICAL STUDIES
14.1 Pediatric Patients with Growth Failure Due to Inadequate Secretion of Endogenous Growth Hormone - An open-label, uncontrolled, multicenter study with Humatrope was conducted in, 314 ...
14.1 Pediatric Patients with Growth Failure Due to Inadequate Secretion of Endogenous Growth Hormone
An open-label, uncontrolled, multicenter study with Humatrope was conducted in, 314 treatment-naive children aged >2 years who had GH deficiency. Patients were treated with HUMATROPE (0.06 mg/kg 3 times per week) for up to 8 years. The efficacy of HUMTROPE was assessed by evaluating height velocity. Mean increase in height velocity from baseline of 3.6 ± 1.9 cm/year to 8.8 ± 2.3 cm/year was achieved by 1 year of treatment. Height velocity remained increased above baseline for a small subset of patients (n=12) who remained on-study up to 8 years during the long-term extension phase (6.3 ± 2.5 cm/year at year 8 of treatment).
14.2 Pediatric Patients with Short Stature Associated with Turner Syndrome
One long-term, randomized, open-label, Canadian multicenter, concurrently controlled study, two long-term, open-label multicenter, historically controlled US studies and one long-term, randomized, US dose-response study were conducted to evaluate the efficacy of HUMATROPE for treatment of short stature due to Turner syndrome.
The randomized, open-label, Canadian study (NCT00191113) compared near-adult height outcomes for HUMATROPE-treated patients to those of a concurrent control group who received no injections. The HUMATROPE-treated patients received a dosage of 0.3 mg/kg/week given in divided doses 6 times per week from a mean age of 11.7 years for a mean duration of 4.7 years. Puberty was induced with a standardized estrogen regimen initiated at 13 years of age for both treatment groups.
In two of the US studies the effect of long-term treatment with Humatrope (0.375 mg/kg/week given in divided doses either 3 times per week or daily) on adult height was determined by comparing adult heights in the treated patients with those of age-matched historical controls with Turner syndrome who received no growth-promoting therapy. Puberty was induced with a standardized estrogen regimen initiated after 14 years of age in one study; in the second study patients treated early (before 11 years of age) were randomized to begin pubertal induction at either age 12 (n=26) or 15 (n=29) years (conjugated estrogens, 0.3 mg escalating to 0.625 mg daily); those whose treatment was initiated after 11 years of age began estrogen replacement after 1 year of treatment with HUMATROPE.
In the third US study, a randomized, blinded dose-response study, patients were treated from a mean age of 10.9 years for a mean duration of 5.5 years with a weekly HUMATROPE dosage of either 0.27 mg/kg or 0.36 mg/kg administered in divided doses 3 or 6 times weekly.
In summary, patients with Turner syndrome (total n=249 from the 4 studies above) treated to adult height achieved average height gains ranging from 5.0 to 8.3 cm. See Table 10.
Table 10: Height Gain Resultsa of Somatropin Studies in Patients with Turner Syndrome a Data shown are mean values.
b RCT: randomized controlled trial; MHT: matched historical controlled trial; RDT: randomized dose-response trial.
c Analysis of covariance (ANCOVA) vs. controls: ANCOVA models with GH (0.27 or 0.36 mg/kg/wk) included covariates of baseline age (all studies), baseline height (all studies), study site (all studies), mid-parental height (all studies) and karyotype (US1 and US2 only), baseline bone age (US2 only) or estrogen (low dose or placebo for US3 only).
d Group A: GH age <11 yr, estrogen is initiated at age 15.
e Group B: GH age <11 yr, estrogen is initiated at age 12.
f Group C: GH age >11 yr, estrogen is initiated at after 12 month of treatment with HUMATROPE.
Study Group Study
DesignbSomatropin Treated Number at Adult Height GH
Age (yr)Estrogen
Age (yr)GH
Duration (yr)Adult Height
Gain (cm)cCanadian RCT 27 11.7 13 4.7 5.4 US 1 MHT 17 9.1 15.2 7.6 7.4 US 2 Ad MHT 29 9.4 15 6.1 8.3 Be MHT 26 9.6 12.3 5.6 5.9 Cf MHT 51 12.7 13.7 3.8 5 US 3 RDT 99 10.9 8-13.5 5.5 5.7 14.3 Pediatric Patients with Idiopathic Short Stature (ISS)
Two randomized, multicenter studies, one placebo-controlled and one dose-response, were conducted in pediatric patients with idiopathic short stature. The diagnosis of idiopathic short stature was made after excluding other known causes of short stature, as well as GH deficiency. The placebo-controlled study enrolled 71 pediatric patients (55 males, 16 females) 9 to 15 years old (mean age 12.4 ± 1.5 years), with short stature, 68 of whom received placebo (n=31) or HUMATROPE (n=37); NCT00191074. Patients were predominately prepubertal (Tanner I, 45%) or in early puberty (Tanner II, 47%) at baseline. In this double-blind study, patients received subcutaneous injections of either HUMATROPE 0.222 mg/kg/week, or placebo given in divided doses 3 times per week until height velocity decreased to ≤1.5 cm/year (“final height”). Final height measurements were available for 33 subjects (22 treated with HUMATROPE, 11 treated with placebo) after a mean treatment duration of 4.4 years (range 0.1-9.1 years).
Results are presented in Table 11. The number of patients whose final height was above the 5th percentile of the general population height standard for age and sex was significantly greater in the HUMATROPE group than the placebo group (41% vs. 0%), as was the number of patients who gained at least 1 SDS unit in height across the duration of the study (50% vs. 0%).
Table 11: Baseline Height Characteristics and Effect of HUMATROPE on Final Height in Placebo-Controlled Studyab a For final height population.
b Abbreviations: BPH=baseline predicted height; CI=confidence interval; FH=final height; NA=not applicable; SDS=standard deviation score.
c Assessed using a one-way analysis of variance (ANOVA) with effect for treatment.
d Placebo n=10 as one patient was missing BPH.
e Assessed using analysis of covariance (ANCOVA) with baseline predicted height SDS as the covariate.
Placebo
(n=11)
Mean (SD)HUMATROPE
(n=22)
Mean (SD)Treatment Effect
Mean
(95%CIb)p-value Baseline height SDS -2.8 (0.6) -2.7 (0.6) NAb 0.77c BPH SDS -2.3 (0.8) -2.1 (0.7) NA 0.53c,d Final height SDS -2.3 (0.6) -1.8 (0.8) 0.51 (0.10, 0.92) 0.017e FHb SDS - baseline height SDS 0.4 (0.2) 0.9 (0.7) 0.51 (0.04, 0.97) 0.034e FH SDS - BPH SDS -0.1 (0.6) 0.3 (0.6) 0.46 (0.02, 0.89) 0.043d,e The dose-response study included 239 pediatric patients (158 males, 81 females), 5 to 15 years old, (mean age 9.8 ± 2.3 years). Mean ± SD baseline characteristics included: height SDS -3.21 ± 0.70, predicted adult height SDS -2.63 ± 1.08, and height velocity SDS -1.09 ± 1.15. All but 3 patients were prepubertal. Patients were randomized to one of three HUMATROPE treatment groups: 0.24 mg/kg/week (n=78); 0.24 mg/kg/week for 1 year, followed by 0.37 mg/kg/week (n=78); and 0.37 mg/kg/week (n=83). Height velocity was assessed in each treatment group during the first 2 years of therapy. After completing the initial 2-year dose-response phase of the study, 50 patients were followed to final height.
After 2 years of treatment, patients who received the HUMATROPE dosage of 0.37 mg/kg/week (n=71) had a significantly greater increase in mean height velocity than patients who received 0.24 mg/kg/week (n=68), (4.04 vs. 3.27 cm/year respectively (ANOVA p=0.003). A total of 12 patients who received 0.37 mg/kg/week and 10 patients who received 0.24 mg/kg/week treatment had missing 2 year treatment data. The mean difference between final height and baseline predicted height was 7.2 cm for patients who received Humatrope 0.37 mg/kg/week and 5.4 cm for patients who received 0.24 mg/kg/week. The results are presented in Table 12. While no patient had height above the 5th percentile in any dosage group at baseline, 82% (n=14) of the 17 patients who received 0.37 mg/kg/week and 47% (n=8) of the 17 patients who received 0.24 mg/kg/week achieved final heights above the 5th percentile of the general population height standards.
Table 12: Idiopathic Short Stature Study Results: Final Height Minus Baseline Predicted Heighta a Abbreviations: FH=final height; PH=predicted height; CI=confidence interval; cm=centimeters.
Placebo-controlled Study
3x per week dosingDose Response Study
6x per week dosingPlacebo
(n=10)HUMATROPE
0.22 mg/kg
(n=22)HUMATROPE
0.24 mg/kg
(n=13)HUMATROPE
0.24/0.37 mg/kg
(n=13)HUMATROPE
0.37 mg/kg
(n=13)FH - Baseline PH
Mean (95% CI), cm-0.7 (-3.6, 2.3) +2.2 (0.4, 3.9) +5.4 (2.8, 7.9) +6.7 (4.1, 9.2) +7.2 (4.6, 9.8) 14.4 Pediatric Patients with Short Stature or Growth Failure in SHOX Deficiency
A randomized, controlled, two-year, three-arm, open-label study was conducted to evaluate the efficacy of HUMATROPE for treatment of short stature in pediatric patients with SHOX deficiency who were not GH–deficient; NCT00190658. A total of 52 patients (24 male, 28 female) with SHOX deficiency, 3 to 12.3 years of age, were randomized to either a HUMATROPE-treated arm (27 patients; mean age 7.3 ± 2.1 years) or an untreated control arm (25 patients; mean age 7.5 ± 2.7 years). To determine the comparability of treatment effect between patients with SHOX deficiency and patients with Turner syndrome, the third study arm enrolled 26 patients with Turner syndrome, 4.5 to 11.8 years of age (mean age 7.5 ± 1.9 years) who received HUMATROPE treatment. All patients were prepubertal at study entry. Patients in the HUMATROPE-treated group(s) received daily subcutaneous injections of 0.05 mg/kg of HUMATROPE, equivalent to 0.35 mg/kg/week. Patients in the untreated group received no injections. One untreated patient discontinued the study during the first year. The results are presented in Table 13; the mean first-year height velocity of treated patients with SHOX deficiency was significantly greater than that of the untreated patients (mean between-group difference = 3.5 [95% CI: 2.8 – 4.2] cm/year, p<0.001).
Table 13: Summary of Results in Patients with SHOX deficiency and Turner Syndrome a Positive values favor HUMATROPE
b p<0.001 (assessed using analysis of covariance [ANCOVA] with covariates of treatment Group, Léri-Weill syndrome, sex and baseline age).
c p<0.001 (assessed using ANCOVA with covariates of treatment group and baseline height [cm]).
d p<0.001 (assessed using ANCOVA with covariates of treatment group and baseline height SDS).
e p=0.003 calculated using Fisher's exact test.
SHOX Deficiency Turner Syndrome Untreated
(n=24)
Mean (SD)HUMATROPE
(n=27)
Mean (SD)Treatment Differencea
Mean (95% CI)HUMATROPE
(n=26)
Mean (SD)Height Velocity (cm/yr) 1st Year 5.2 (1.1) 8.7 (1.6)b +3.5 (2.8, 4.2) 8.9 (2.0) 2nd Year 5.4 (1.2) 7.3 (1.1)b +2.0 (1.3, 2.6) 7.0 (1.1) Height Gain (cm) Baseline to 1st Year 5.4 (1.2) 9.1 (1.5)c +3.7 (2.9, 4.5) 8.9 (1.9) Baseline to 2nd Year 10.5 (1.9) 16.4 (2.0)c +5.8 (4.6, 7.1) 15.7 (2.7) Height SDS Gain Baseline to 1st Year 0.1 (0.5) 0.7 (0.5)d +0.5 (0.3, 0.8) 0.8 (0.5) Baseline to 2nd Year 0.2 (0.5) 1.2 (0.7)d +1.0 (0.7, 1.3) 1.2 (0.7) Patients with height SDS > -2.0 at 2 years 1 (4%) 11 (41%)e -- 8 (31%) 14.5 Pediatric Patients with Short Stature Born Small for Gestational Age (SGA) Who Fail to Demonstrate Catch-up Growth by Age 2 - 4 Years
The height increases would be considered similar if the lower bound of the 95% confidence interval (CI) for the mean difference between the groups (IAD – FHD) was greater than -0.5 height SDS. A 2-year, open-label, multicenter, European study enrolled 193 prepubertal, non-GH deficient children with mean chronological age 6.8 ± 2.4 years (range: 3 to 12.3); NCT00191529. Study entry criteria included birth weight <10th percentile and/or birth length SDS <-2 for gestational age, and height SDS for chronological age ≤-3. Exclusion criteria included syndromal conditions (e.g., Turner syndrome), chronic disease (e.g., diabetes mellitus), and active tumors. The primary objective was to compare the increase from baseline in height SDS after 1 year of treatment when HUMATROPE is administered according to an individually adjusted dose (IAD) regimen with a fixed high dose (FHD) regimen. Patients were randomized to either a FHD (0.067 mg/kg/day [0.47 mg/kg/week]; n=99) or an IAD treatment group (n=94). The initial HUMATROPE dosage in the IAD treatment group was 0.035 mg/kg/day (0.25 mg/kg/week). The dosage was increased to 0.067 mg/kg/day in those patients in the IAD group whose 1-year height gain predicted at Month 3 was <0.75 height SDS (n=40) or whose actual height gain measured at Year 1 was <0.75 height SDS (n=11).
The results are presented in Table 14, The increase from baseline in height SDS in the IAD group was non-inferior to that in the FHD group at Year 1 (mean between-group difference = -0.3 SDS [95% CI: -0.4, -0.2 SDS]). The results were similar when children who entered puberty during the study were removed from the analysis. Data is shown for the efficacy analysis population that included all patients who received at least 1 dose of HUMATROPE and had at least 1 post randomization height measurement. Approximately 85% of the randomized patients completed 2 years of therapy.
Table 14: Results for Height SDS and Change from Baseline in Height SDS at Year 1 and Year 2 After HUMATROPE Treatment of Short Children Born SGA Who Fail to Demonstrate Catch-up Growtha a Abbreviations: IAD=individually adjusted dose; FHD=fixed high dose; SD=standard deviation; SDS=standard deviation score
b Least squares mean difference ± standard error and 95% confidence interval based on ANCOVA model with treatment and gender as fixed effects, and baseline height SDS, baseline chronological age, baseline bone age, and mid-parental target height SDS as covariates.
c Only children with actual height measurements were included in the Year 1 and Year 2 analyses.
IAD Group
0.035 to 0.067 mg/kg/day
Mean (SD)FHD Group
0.067 mg/kg/day
Mean (SD)IAD – FHD Group Difference ± SE
(95% CI)
bBaseline (n=86)
-3.9 (0.6)(n=93)
-3.9 (0.7)-0.0 ± 0.1
(-0.2, 0.2)
Year 1
Height SDS
Change from baseline(n=86)c
-3.0 (0.7)
0.9 (0.4)(n=93)c
-2.7 (0.7)
1.1 (0.4)-0.3 ± 0.1
(-0.4, -0.2)
p-value <0.001Year 2
Height SDS
Change from baseline(n=82)c
-2.5 (0.8)
1.4 (0.5)(n=88)c
-2.2 (0.7)
1.6 (0.5)-0.3 ± 0.1
(-0.4, -0.1)
An open-label, multicenter, single arm study was conducted in France, during which 35 prepubertal, non-GH deficient children were treated for 2 years with HUMATROPE 0.067 mg/kg/day (0.47 mg/kg/week). Mean chronological age at baseline was 9.3 ± 0.9 years (range: 6.7 to 10.8). Additional study entry criteria included birth length SDS <-2 or <3rd percentile for gestational age, and height SDS for chronological age <-2. Exclusion criteria included syndromal conditions (e.g., Turner syndrome), and chronic disease (e.g., diabetes mellitus). All 35 patients completed the study. Mean height SDS increased from a baseline value of -2.7 (SD 0.5) to -1.5 (SD 0.6) after 2 years of HUMATROPE treatment.
Close14.6 Adult Patients with Growth Hormone Deficiency
Two multicenter studies in patients with adult-onset GH deficiency (n=98) and two studies in patients with childhood-onset GH deficiency (n=67) were designed to assess the effects of replacement therapy with HUMATROPE. Adult-onset patients and childhood-onset patients differed by diagnosis (organic vs. idiopathic pituitary disease), body size (average vs. small [mean height (171 cm vs 161 cm) and weight (85 kg vs 64 kg)]), and age (mean 44 vs. 29 years).Each study included a 6-month randomized, blinded, placebo-controlled phase, during which approximately half of the patients received placebo injections, while the other half received HUMATROPE injections. The HUMATROPE dosages for all studies were identical: 1 month of treatment at 0.00625 mg/kg/day followed by 0.0125 mg/kg/day for the next 5 months. The 6-month, double-blind phase was followed by 12 months of open-label HUMATROPE treatment for all patients. The primary efficacy measures were body composition (lean body mass and fat mass) and lipid parameters [Total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL)]. Lean body mass was determined by bioelectrical impedance analysis (BIA), validated with potassium 40. Body fat was assessed by BIA and sum of skinfold thickness. Lipid subfractions were analyzed by standard assay methods in a central laboratory.
In a study with patients with adult-onset GH deficiency, HUMATROPE treatment (vs. placebo) resulted in an increase in mean lean body mass (2.59 vs. -0.22 kg, p<0.001) and a decrease in body fat (-3.27 vs. 0.56 kg, p<0.001). Similar changes were seen in childhood-onset GH deficient patients. These changes in lean body mass persisted throughout the 18-month period for both the adult-onset and childhood-onset groups; the changes in fat mass persisted in the childhood-onset group. Serum concentrations of HDL cholesterol which were low at baseline (mean, 31.0 mg/dL and 33.9 mg/dL in adult-onset (n=46) and childhood-onset (n=30) patients, respectively) had increased by the end of 18 months of HUMATROPE treatment (mean change of 13.7 and 11.1 mg/dL for the adult-onset (n=40) and childhood-onset (n=21) groups, respectively p<0.001; there was no adjustment for missing data). Total cholesterol and LDL did not show significant difference from baseline results by the end of 18 months of HUMATROPE treatment.
In an additional 2-year, open-label, randomized study 149 patients with childhood-onset GH deficiency who had completed pediatric somatropin therapy, had attained final height (height velocity <1 cm/yr) and were confirmed to be GH-deficient as young adults, were randomized to receive HUMATROPE 0.0125 mg/kg/day (n=59), HUMATROPE 0.025 mg/kg/day (n=58), or no treatment (control) (n=32). Total bone mineral content (BMC) increased by 5.2% ± 3.9% in the control group (n=28 for those with BMC measurements), 7.0% ± 7.2% in the HUMATROPE 0.025 mg/kg/day group (n=51) and 8.0% ± 8.9% in the HUMATROPE 0.0125 mg/kg/day group (n=51). For the treatment effect versus control group, p=0.012 (ANOVA); there was no statistically significant difference between the 2 HUMATROPE dose groups. A significant overall treatment effect (ANOVA, p=0.037) was seen for the percentage change in lumbar spine BMC, but not for femoral neck or hip.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - HUMATROPE (somatropin) for injection is a white lyophilized powder available in the following cartridge sizes in Table 15: Table 15: Components of Humatrope ...
16.1 How Supplied
HUMATROPE (somatropin) for injection is a white lyophilized powder available in the following cartridge sizes in Table 15:
Table 15: Components of Humatrope Kits NDC Kit HUMATROPE Diluent NDC 0002-8147-01 Cartridge Kit 6 mg Single-Patient-Use cartridge (gold) prefilled syringe of Diluent for HUMATROPE NDC 0002-8148-01 Cartridge Kit 12 mg Single-Patient-Use cartridge (teal) prefilled syringe of Diluent for HUMATROPE NDC 0002-8149-01 Cartridge Kit 24 mg Single-Patient-Use cartridge (purple) prefilled syringe of Diluent for HUMATROPE Close16.2 Storage and Handling
Cartridges
Refrigerate cartridges of HUMATROPE and Diluent for HUMATROPE at 36° to 46°F (2° to 8°C). Avoid freezing Diluent for HUMATROPE. Store in the original carton to protect HUMATROPE from light.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use). Neoplasms – Advise childhood cancer survivors/caregivers that individuals treated with brain/head radiation ...
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
- Neoplasms – Advise childhood cancer survivors/caregivers that individuals treated with brain/head radiation are at increased risk of secondary neoplasms and as a precaution need to be monitored for recurrence. Advise patients/caregivers to report marked changes in behavior, onset of headaches, vision disturbances and/or changes in skin pigmentation or changes in the appearance of pre-existing nevi.
- Fluid Retention - Advise patients that fluid retention during HUMATROPE replacement therapy in adults may frequently occur. Inform patients of the clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) and to report to their healthcare provider any of these signs or symptoms that occur during treatment with HUMATROPE.
- Pancreatitis - Advise patients/caregivers that pancreatitis may develop and to report to their healthcare provider any new onset of abdominal pain.
- Hypoadrenalism - Advise patients/caregivers who have or who are at risk for pituitary hormone deficiency(s) that hypoadrenalism may develop and to report to their healthcare provider if they experience hyperpigmentation, extreme fatigue, dizziness, weakness, or weight loss.
- Hypothyroidism - Advise patients/caregivers that undiagnosed/untreated hypothyroidism may prevent an optimal response to HUMATROPE. Advise patients/caregivers they may require periodic thyroid function tests.
- Intracranial Hypertension - Advise patients/caregivers to report to their healthcare provider any visual changes, headache, and nausea and/or vomiting.
- Hypersensitivity Reactions – Advise patients/caregivers that serious systemic hypersensitivity reactions (anaphylaxis and angioedema) are possible and that prompt medical attention should be sought if an allergic reaction occurs.
- Glucose Intolerance/ Diabetes Mellitus – Advise patients/caregivers that new onset impaired glucose intolerance/diabetes mellitus or exacerbation of preexisting diabetes mellitus can occur and monitoring of blood glucose during treatment with HUMATROPE may be needed.
- Administration – Counsel patients and parents that they should never share a HumatroPen with another person, even if the needle is changed. Sharing of the pen between patients may pose a risk of transmission of infection.
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License Number 1891Copyright ©, 1987, 2023, Eli Lilly and Company. All rights reserved.
HTR-0005-USPI-20231208
Close -
Humatrope Cartrige Kit Instuctions for UseInstructions for Use - HUMATROPE® (HU-ma-trope) (somatropin) For injection, for subcutaneous use - Single-Patient-Use Cartridge Kit - HUMATROPE cartridges are only to be used with ...
Instructions for Use
HUMATROPE® (HU-ma-trope)
(somatropin)
For injection, for subcutaneous use
Single-Patient-Use Cartridge Kit
HUMATROPE cartridges are only to be used with HumatroPen® injection devices.
Read this Instructions for Use before you start using HUMATROPE Cartridge Kit and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Your healthcare provider should show you how to prepare, mix, measure, and inject HUMATROPE the right way before you use it for the first time. Ask your healthcare provider if you have any questions.
How should I store HUMATROPE?
- Store HUMATROPE in the refrigerator between 36°F to 46°F (2°C to 8°C) before and after mixing.
- Do not freeze.
- Store HUMATROPE in the original carton to protect from light.
- HUMATROPE that has been mixed and is in liquid form must be used within 28 days. Throw away any mixed HUMATROPE left over after 28 days.
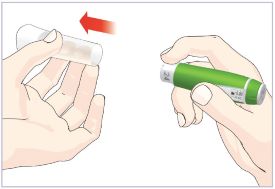
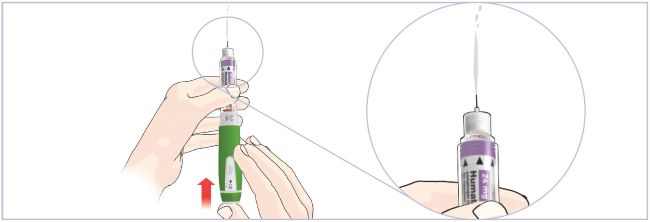
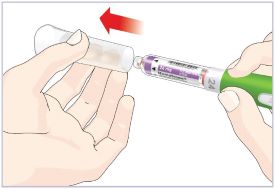
- Before giving an injection, check the expiration date on the Cartridge. Do not use the Cartridge if it has expired (See Figure A).
Important:
- HUMATROPE Cartridges should not be used if you are allergic to metacresol or glycerin.
- The HUMATROPE Cartridge is not recommended for use by blind or visually impaired individuals without the help of a sighted individual trained on how to use it.
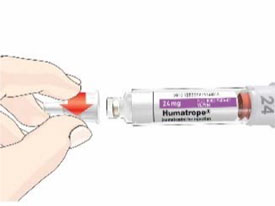
HUMATROPE Cartridge Kit (See Figure A)
- 1 Cartridge with 6 mg, 12 mg, or 24 mg of powdered HUMATROPE
- 1 prefilled syringe with Diluent (the liquid used to mix the powdered HUMATROPE)
Note: There are 3 strengths of HUMATROPE Cartridges that have different amounts of HUMATROPE (6 mg, 12 mg, or 24 mg). Make sure that you have the Cartridge that your healthcare provider prescribed.
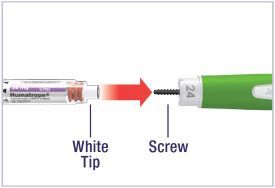
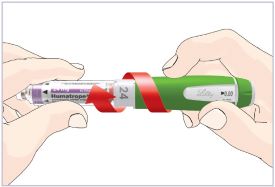
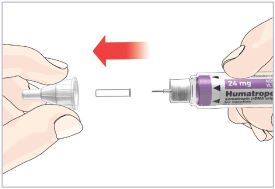
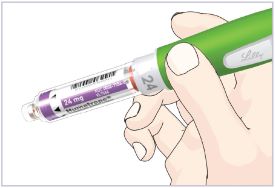
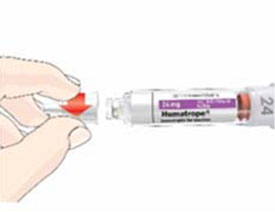
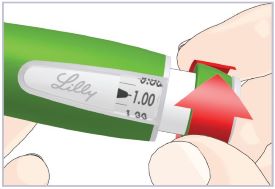
Mixing the HUMATROPE in the Cartridge
Only use the prefilled Diluent Syringe that comes with your HUMATROPE Cartridge Kit to mix the HUMATROPE in the Cartridge. Do not use the diluent that comes in the HUMATROPE vial box, or any other liquid.
HUMATROPE Cartridge Kit mixing Instructions
Only use a HUMATROPE Cartridge Kit to prepare the HUMATROPE Cartridge.
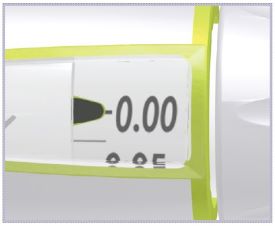
*Note: The liquid (Diluent) should be clear, colorless, and free of particles and is shown as blue in the figure
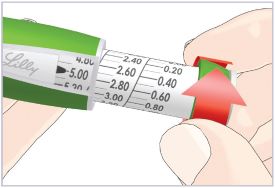
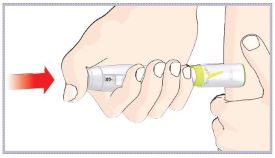
(See Figure A).Talk to your healthcare provider about the right way to use your HUMATROPE Cartridge Kit and HumatroPen injection device, the right sites to inject HUMATROPE, and how to rotate your injection sites.
Disposing of used Diluent Syringes:
- Put your used Diluent Syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
HUMATROPE® and HumatroPen® are registered trademarks of Eli Lilly and Company.
Copyright © 2019, 2023 Eli Lilly and Company. All rights reserved.
Revised December 2023
Manufactured by: Eli Lilly and Company, Indianapolis, IN 46285, USA
US License Number 1891
www.HUMATROPE.com
HTRCART-0002-IFU-20231208
Close -
Humatrope 6 mg HumatroPen Instuctions for UseInstructions for Use - HumatroPen® 6 mg - (HU-ma-tro-Pen 6 mg) HUMATROPE® Delivery System Device - for use with - HUMATROPE® (somatropin) 6 mg Cartridges - 6 mg - Section 1 ...
Instructions for Use
HumatroPen® 6 mg
(HU-ma-tro-Pen 6 mg)
HUMATROPE® Delivery System Device
for use with
HUMATROPE® (somatropin) 6 mg Cartridges
Section 1: Read Section 1 completely before you move on to Section 2.
What you need to know about HumatroPen® 6 mg
Read this Instructions for Use including the “Commonly asked questions” section, at the end of this Instructions for Use, before you start using HumatroPen 6 mg and each time you get a new Pen. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Your healthcare provider should show you how to use HumatroPen (Pen) the right way before you use it for the first time. Ask your healthcare provider if you have any questions.
 If you are having problems using the HumatroPen 6 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
If you are having problems using the HumatroPen 6 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Introduction
Intended Use:
- The HumatroPen 6 mg is a reusable pen-injector designed for self-administration of human growth hormone. The pen-injector is intended for use with the specified HUMATROPE 6 mg Cartridges and single use, detachable and disposable Pen Needles (supplied separately). Your healthcare provider will prescribe the HUMATROPE dose and Pen that is right for you or your child. Do not change your dose or use another type of Pen unless your healthcare provider tells you to.
- If your healthcare provider changes the prescribed cartridge strength from the 6 mg HUMATROPE Cartridge to the 12 mg or 24 mg HUMATROPE Cartridge, you will need to get a new HumatroPen to match the new cartridge strength.
Contraindications:
There are no known contraindications for HumatroPen 6 mg.
Important information about HumatroPen 6 mg:
- Do not use the Pen if any part of the Pen or Cartridge looks broken or damaged. Contact your healthcare provider.
- Make sure that you have a 6 mg HUMATROPE Cartridge to match the HumatroPen 6 mg. If the strength on the label for the Cartridge and the Pen do not match, do not use them and contact your healthcare provider. It is important to use the correct Pen and Cartridge to make sure that you inject the correct dose of HUMATROPE.
- Do not use the HUMATROPE Cartridge past the expiration date.
- Follow the instructions in Section 2 only to set up a new Cartridge before you use it for the first time.
- Follow the instructions in Section 3 of this Instructions for Use for every injection.
- Do not transfer the contents of the HUMATROPE Cartridge to a syringe.
- Do not share your HumatroPen 6 mg or Pen Needles with anyone else, even if the Needle has been changed. You may give other people a serious infection or get a serious infection from them.
- The HumatroPen 6 mg is not recommended for use by blind or visually impaired individuals without the help of a sighted individual trained on how to use it.
Information about Needles used with HumatroPen 6 mg:
- Pen Needles are not included with HumatroPen. You may need a prescription to get the Needles from your pharmacist.
- Becton, Dickinson and Company Pen Needles are recommended for use with the HumatroPen 6 mg.
- Ask your healthcare provider what size Needle is best for you to use.
- Use of the Hidden Needle Cover with your HumatroPen is optional.
- When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
- Follow your healthcare provider's instructions about how to safely handle Needles.
- Always remove the Pen Needle after each injection and use a new Needle with each injection. This helps reduce the risk of infection, prevent leakage of HUMATROPE, reduce air bubbles, and reduce Needle clogs.
- For information on the right way to throw away (dispose of) used Needles, see “Disposing of used Needles” at the end of this Instructions for Use.
How do I care for HumatroPen 6 mg?
- Soiled parts including the Hidden Needle Cover can be cleaned with a damp cloth. Do not use alcohol or any other cleaning product.
- Keep dry.
- Do not apply oil or any other lubricant.
How do I store HumatroPen 6 mg with an attached cartridge?
- Store HumatroPen 6 mg between -40°C (-40°F) to 70°C (158°F) without the Humatrope 6 mg Cartridge.
- After attaching a prepared HUMATROPE cartridge to your HumatroPen 6 mg, store it in the storage case in the refrigerator between 36°F to 46°F (+2°C to +8°C) until it is time for your next injection.
- Before injecting a dose of HUMATROPE, take the Pen out of the refrigerator and let it sit for 10 minutes to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort. Do not leave the HumatroPen 6 mg and HUMATROPE cartridge out of the refrigerator for more than 30 minutes a day.
- HUMATROPE Cartridges attached to a Pen can be stored in the refrigerator for up to 28 days. Do not use a prepared Cartridge after 28 days.
- Do not freeze.
- Do not store the Pen with the Needle attached.
- Protect HUMATROPE Cartridges attached to the Pen from light during storage.
When do I need to replace and dispose my HumatroPen?
Do not use your Pen for more than 3 years after first use or past the Use-by date on the carton, whichever comes first.
The expired Pen may be discarded in your household trash after you have removed the Needle.
Record the date the Pen was first used here: __ / __ / __.
Record the Use-by date from your carton here: __ / __ / __.
Contact your healthcare provider if you need a new HumatroPen 6 mg, or when the Pen has been used for 3 years.
 Please see the accompanying complete Humatrope Patient Information Sheet.
Please see the accompanying complete Humatrope Patient Information Sheet.
For more information, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Section 2: Read Section 2 only after you have read Section 1.
Getting Started
- Be sure to follow the reconstitution (mixing) directions in the HUMATROPE Cartridge Kit.
- Perform the New Cartridge Set-up only 1 time at the beginning of each new Cartridge preparation.
- For daily use, do not repeat the 1 time only New Cartridge Set-up. If you repeat the 1 time only New Cartridge Set-up, you may run out of HUMATROPE early.
New Cartridge Set-up
Important Notes:
- The Pen must be set-up before injecting the first dose from each new 6 mg Cartridge.
- Setting up the new Cartridge is important to remove large air bubbles that may be present after mixing the HUMATROPE 6 mg Cartridge.
- If liquid is not seen at the Needle tip after you have tried several times to remove air from the new Cartridge as instructed in Step 2D, contact your healthcare provider or Lilly at 1-800-545-5979.
Step 2E – Continue on to Daily Use
- Do not repeat the New Cartridge Set-up before each dose.
- Leave the Cartridge attached and do not remove it until the Cartridge is empty.
- If you are ready to inject the first dose and you have finished setting up your Pen with a new Cartridge, go to Section 3, Step 3C.
- If you are using the Hidden Needle Cover with HumatroPen 6 mg, read the Hidden Needle Cover Instructions for Use.
Section 3: Now that you have done the 1 time only New Cartridge Set-up, follow Section 3 for each of your daily injections.
- Remove the prepared Pen with the Cartridge attached from your refrigerator.
- Let the Pen with Cartridge attached stand at room temperature for 10 minutes before injecting.
Daily Use
Before your injection: Take the Pen out of the refrigerator and let it sit for 10 minutes before injecting to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort.
Important Note: Talk to your healthcare provider about the right way to use the Pen, and the optional Hidden Needle Cover, the right sites to inject HUMATROPE, and how to rotate your injection sites. When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
Step 3C – Dial and inject the dose
Step 3D – Remove and dispose of the Needle
Important Note: Do not remove this Cartridge from the Pen until the Cartridge is empty or needs to be replaced to help prevent you from giving the wrong dose.
Step 3E – Store Pen and Cartridge for next use
- See, “How do I store HumatroPen 6 mg?” in Section 1 of this Instructions for Use for more information.
- When it is time for the next scheduled dose, go to Section 3, and repeat Steps 3A through 3D.
- When using the Hidden Needle Cover with HumatroPen 6 mg, repeat steps 1 to 10 of the Hidden Needle Cover Instructions for Use.
Commonly asked questions:
-
Do I need to perform the New Cartridge Setup before every dose?
- No. You only need to perform the New Cartridge Set-up 1 time for each Cartridge, just before a new Cartridge is used for the first time.
- The purpose of the New Cartridge Set-up is to make sure the HumatroPen 6 mg with an attached HUMATROPE 6 mg Cartridge are ready to use.
- If you repeat the New Cartridge Set-up before each routine dose, you may run out of HUMATROPE early. The small amount of medicine used in the New Cartridge Set-up will not affect the amount of HUMATROPE in the Cartridge.
-
What should I do if the Cartridge label and Pen do not match?
- Do not use the Pen if the Cartridge strength on the HUMATROPE Cartridge label does not match the number on the Pen's front housing. This is important to make sure the correct dose of HUMATROPE is given.
- Contact your healthcare provider for a replacement.
-
What should I do if the HUMATROPE is not clear?
- Do not use the Pen if the liquid is cloudy or contains particles. Contact your healthcare provider or call Lilly at 1-800-545-5979 or go to www.humatrope.com.
-
Why are there air bubbles in the Cartridge?
- Air bubbles may remain in the Cartridge after mixing.
- If the Pen is stored with a Needle attached, air bubbles may form in the Cartridge.
Do not store the Pen with a Needle attached. - Follow the instructions in Section 2, Step 2D (Remove air from new Cartridge) to remove air bubbles from the Cartridge.
- It is normal to see small air bubbles. It will not cause any harm or affect the dose.
-
What should I do if the screw does not move out when there is no Cartridge attached to the Pen?
- The screw may not move out when you push the Injection Button unless there is a Cartridge in the Pen. This allows you to easily push the screw into the Pen body when replacing a Cartridge.
- After a Cartridge is attached, the screw will move out when the Injection Button is pushed.
-
What should I do if I cannot attach the Cartridge to the Pen body?
- Check that the Cartridge is not damaged or broken.
- Carefully line up the Cartridge with the Pen body and screw them together until secure. If the Cartridge and Pen cannot be screwed together, contact your healthcare provider.
-
Why is it hard to push the Injection Button when I try to inject the dose?
- The Needle may be clogged. Try attaching a new Needle.
- Pushing the Injection Button down quickly may make the Injection Button harder to push. Pushing the Injection Button more slowly may make it easier.
- Using a larger Needle will make it easier to push the Injection Button during injection. Ask your healthcare provider which Needle is best for you.
- The Injection Button may become harder to push if the inside of the Pen gets dirty with HUMATROPE, food, drink, or other materials.
-
What should I do if the dose knob does not go to zero (0) when I inject the dose?
- This can happen if the HUMATROPE Cartridge does not have enough HUMATROPE left in it for the full dose.
- At the end of the injection, the number in the dose window should be 0.00. If you see any number other than “0.00”, this is the amount of HUMATROPE you did not receive. Write this number down and follow the steps below to get the rest of your dose.
- To get the rest of your dose:
- -
- Remove the Needle and empty Cartridge from the Pen.
- -
- Insert a new Cartridge, attach a new Needle, and remove air from the new Cartridge (see Steps 2A through 2D).
- -
- Complete your dose by injecting only the amount you did not receive (see Step 3C).
- -
- If you still do not think you received your full dose, do not take another dose. Call Lilly at 1-800-545-5979 or your healthcare provider for assistance.
-
What should I do if I see HUMATROPE leaking from the Needle after I have finished the injection?
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
- -
- The full dose may not have been delivered. Do not inject another dose. Talk with your healthcare provider for assistance.
- -
- To prevent this, for the next dose, firmly push and hold the Injection Button in and slowly count to 5 (see Section 3, Step 3C).
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
-
How can I tell when the injection is complete?
- The injection is complete when:
- -
- You place your thumb on the Injection Button, then slowly and firmly push the Injection Button until it stops moving.
- -
- Continue to hold the Injection Button for 5 seconds, then remove the Needle from the skin.
- -
- You will see 0.00 in the center of the dose window.
- The injection is complete when:
If you have any questions or problems with your HumatroPen 6 mg, contact Lilly at 1-800-545-5979 or your healthcare professional for assistance.
Report all MEDICAL DEVICE COMPLAINTS or UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to Eli Lilly and Company, by contacting them at 1-800-545-5979.
You are also encouraged to report all UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to the U.S. FOOD & DRUG Administration (US FDA) by:
- Visiting the MedWatch web page on reporting an adverse event or serious problem to FDA at (https://www.fda.gov/safety/reporting-serious-problems-fda/how-consumers-can-report-adverse-event-or-serious-problem-fda) or
- Calling FDA’s toll-free information line at 1-800-INFO-FDA (1-888-463-6332).
Disposing of used Needles:
- Put your used Needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose Needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles. Do not reuse or share your Needles with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

Eli Lilly and Company
Pharmaceutical Delivery Systems
Lilly Corporate Center
Indianapolis, IN 46285, USA
US License Number 1891
Eli Lilly Nederland B.V.
Papendorpseweg 83
3528 BJ UTRECHT
The NetherlandsHUMATROPE® and HumatroPen® 6 mg are registered trademarks of Eli Lilly and Company.
The HumatroPen® 6 mg meets the current dose accuracy and functional requirements of ISO 11608-1 and is for use with HUMATROPE® 6 mg Cartridges only. Copyright © 2009, 2023, Eli Lilly and Company. All rights reserved.
Revised December 2023Non-harmonized Symbol Glossary1
1 Glossary includes non-harmonized symbols used in the labeling.
2 Symbol used only on the carton.HTRPEN6-0005-IFU-20231208
Close -
Humatrope 12 mg HumatroPen Instuctions for UseInstructions for Use - HumatroPen® 12 mg - (HU-ma-tro-Pen 12 mg) HUMATROPE® Delivery System Device - for use with - HUMATROPE® (somatropin) 12 mg Cartridges - 12 mg - Section ...
Instructions for Use
HumatroPen® 12 mg
(HU-ma-tro-Pen 12 mg)
HUMATROPE® Delivery System Device
for use with
HUMATROPE® (somatropin) 12 mg Cartridges
Section 1: Read Section 1 completely before you move on to Section 2.
What you need to know about HumatroPen® 12 mg
Read this Instructions for Use including the “Commonly asked questions” section, at the end of this Instructions for Use, before you start using HumatroPen 12 mg and each time you get a new Pen. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Your healthcare provider should show you how to use HumatroPen (Pen) the right way before you use it for the first time. Ask your healthcare provider if you have any questions.
 If you are having problems using the HumatroPen 12 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
If you are having problems using the HumatroPen 12 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Introduction
Intended Use:
- The HumatroPen 12 mg is a reusable pen-injector designed for self-administration of human growth hormone. The pen-injector is intended for use with the specified HUMATROPE 12 mg Cartridges and single use, detachable and disposable pen needles (supplied separately). Your healthcare provider will prescribe the HUMATROPE dose and Pen that is right for you or your child. Do not change your dose or use another type of pen unless your healthcare provider tells you to.
- If your healthcare provider changes the prescribed cartridge strength from the 12 mg HUMATROPE Cartridge to the 6 mg or 24 mg HUMATROPE Cartridge, you will need to get a new HumatroPen to match the new cartridge strength.
Contraindications:
There are no known contraindications for HumatroPen 12 mg.
Important information about HumatroPen 12 mg:
- Do not use the Pen if any part of the Pen or Cartridge looks broken or damaged. Contact your healthcare provider.
- Make sure that you have a 12 mg HUMATROPE Cartridge to match the HumatroPen 12 mg. If the strength on the label for the Cartridge and the Pen do not match, do not use them and contact your healthcare provider. It is important to use the correct Pen and Cartridge to make sure that you inject the correct dose of HUMATROPE.
- Do not use the HUMATROPE Cartridge past the expiration date.
- Follow the instructions in Section 2 only to set up a new Cartridge before you use it for the first time.
- Follow the instructions in Section 3 of this Instructions for Use for every injection.
- Do not transfer the contents of the HUMATROPE Cartridge to a syringe.
- Do not share your HumatroPen 12 mg or Pen Needles with anyone else, even if the Needle has been changed. You may give other people a serious infection or get a serious infection from them.
- The HumatroPen 12 mg is not recommended for use by blind or visually impaired individuals without the help of a sighted individual trained on how to use it.
Information about Needles used with HumatroPen 12 mg:
- Pen Needles are not included with HumatroPen. You may need a prescription to get the Needles from your pharmacist.
- Becton, Dickinson and Company Pen Needles are recommended for use with the HumatroPen 12 mg.
- Ask your healthcare provider what size Needle is best for you to use.
- Use of the Hidden Needle Cover with your HumatroPen is optional.
- When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
- Follow your healthcare provider's instructions about how to safely handle Needles.
- Always remove the Pen Needle after each injection and use a new Needle with each injection. This helps reduce the risk of infection, prevent leakage of HUMATROPE, reduce air bubbles, and reduce Needle clogs.
- For information on the right way to throw away (dispose of) used Needles see “Disposing of used Needles” at the end of this Instructions for Use.
How do I care for HumatroPen 12 mg?
- Soiled parts including the Hidden Needle Cover can be cleaned with a damp cloth. Do not use alcohol or any other cleaning product.
- Keep dry.
- Do not apply oil or any other lubricant.
How do I store HumatroPen 12 mg with an attached cartridge?
- Store HumatroPen 12 mg between -40°C (-40°F) to 70°C (158°F) without the Humatrope 12 mg Cartridge.
- After attaching a prepared HUMATROPE cartridge to your HumatroPen 12 mg, store it in the storage case in the refrigerator between 36°F to 46°F (+2°C to +8°C) until it is time for your next injection.
- Before injecting a dose of HUMATROPE, take the Pen out of the refrigerator and let it sit for 10 minutes to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort. Do not leave the HumatroPen 12 mg and HUMATROPE cartridge out of the refrigerator for more than 30 minutes a day.
- HUMATROPE Cartridges attached to a Pen can be stored in the refrigerator for up to 28 days. Do not use a prepared Cartridge after 28 days.
- Do not freeze.
- Do not store the Pen with the Needle attached.
- Protect HUMATROPE Cartridges attached to the Pen from light during storage.
When do I need to replace and dispose my HumatroPen?
Do not use your pen for more than 3 years after the first use or past the Use-by date on the carton, whichever comes first.
The expired Pen may be discarded in your household trash after you have removed the Needle.
Record the date the Pen was first used here: __ / __ / __.
Record the Use-by date from your carton here: __ / __ / __.
Contact your healthcare provider if you need a new HumatroPen 12 mg, or when the Pen has been used for 3 years.
 Please see the accompanying complete Humatrope Patient Information Sheet.
Please see the accompanying complete Humatrope Patient Information Sheet.
For more information, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Section 2: Read Section 2 only after you have read Section 1.
Getting Started
- Be sure to follow the reconstitution (mixing) directions in the HUMATROPE Cartridge Kit.
- Perform the New Cartridge Set-up only 1 time at the beginning of each new Cartridge preparation.
- For daily use, do not repeat the 1 time only New Cartridge Set-up. If you repeat the 1 time only New Cartridge Set-up, you may run out of HUMATROPE early.
New Cartridge Set-up
Important Notes:
- The Pen must be set-up before injecting the first dose from each new 12 mg Cartridge.
- Setting up the new Cartridge is important to remove large air bubbles that may be present after mixing the HUMATROPE 12 mg Cartridge.
- If liquid is not seen at the Needle tip after you have tried several times to remove air from the new Cartridge as instructed in Step 2D, contact your healthcare provider or Lilly at 1-800-545-5979.
Step 2E – Continue on to Daily Use
- Do not repeat the New Cartridge Set-up before each dose.
- Leave the Cartridge attached and do not remove it until the Cartridge is empty.
- If you are ready to inject the first dose and you have finished setting up your Pen with a new Cartridge, go to Section 3, Step 3C.
- If you are using the Hidden Needle Cover with HumatroPen 12 mg, read the Hidden Needle Cover Instructions for Use.
Section 3: Now that you have done the 1 time only New Cartridge Set-up, follow Section 3 for each of your daily injections.
- Remove the prepared Pen with the Cartridge attached from your refrigerator.
- Let the Pen with Cartridge attached stand at room temperature for 10 minutes before injecting.
Daily Use
Before your injection: Take the Pen out of the refrigerator and let it sit for 10 minutes before injecting to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort.Step 3B – Attach a new disposable Pen Needle for each injection.
- Remove the paper tab from the end of the Outer Cap.
- Push the Needle straight onto the 12 mg Cartridge and turn it until secure.
- Pull off the Outer Cap and the Inner Cap.
- Keep the Outer Cap to remove the Needle after the injection.
Important Note: Talk to your healthcare provider about the right way to use the Pen, and the optional Hidden Needle Cover, the right sites to inject HUMATROPE, and how to rotate your injection sites. When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
Step 3C – Dial and inject the dose
- Turn the dose knob to the prescribed dose.
Confirm the dose dialed matches the prescribed dose.
Your dose may be different than the dose shown in the figure above.- If you turn past the prescribed dose, you can correct the dose by turning down to the right dose.
- The dose knob clicks as you turn it. Do not dial your dose by counting clicks because you may dial the wrong dose.
- Injections can be given under the skin (subcutaneous) in the following areas:
- -
- Abdomen: above, below, or either side of the belly button (navel)
- -
- Front of the upper thighs
- -
- Upper, outer buttocks
- -
- Back of the arms above the elbow and below the shoulder
- Talk to your healthcare provider about the right way to use HumatroPen, the right areas to give your injection, and injection site rotation.
- Insert the Needle at a 90 degree angle or as shown by your healthcare provider.
- Place your thumb on the injection button, then slowly and firmly push the Injection Button until it stops moving.
- Continue to hold the Injection Button for 5 seconds, then remove the Needle from the skin.
- Check to make sure you see a 0.00 in the dose window. This means that the complete dose was injected.
Important Note:
It is possible to set a dose larger than the amount of HUMATROPE left in the Cartridge.
- At the end of the injection, the number in the dose window should be 0.00. If you see any number other than “0.00”, this is the amount of HUMATROPE you did not receive. Write this number down and follow the steps below to get the rest of your dose.
- Remove the Needle and empty Cartridge from the Pen.
- Repeat Steps 2A through 2D.
- Complete your dose by injecting only the amount you did not receive.
- If you still do not think you received your full dose, do not take another dose. Call Lilly at 1-800-545-5979 or call your healthcare provider for assistance.
Step 3D – Remove and dispose of the Needle
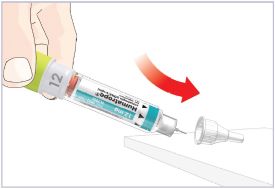
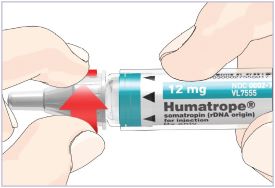
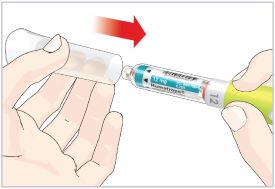
- Remove the capped Needle. Follow the instructions in the “Disposing of used Needles” section at the end of this Instructions for Use to dispose of the Needle.
- Replace the Pen Cap.
Important Note: Do not remove this Cartridge from the Pen until the Cartridge is empty or needs to be replaced to help prevent you from giving the wrong dose.
Step 3E – Store Pen and Cartridge for next use
- See, “How do I store HumatroPen 12 mg?” in Section 1 of this Instructions for Use for more information.
- When it is time for the next scheduled dose, go to Section 3, and repeat Steps 3A through 3D.
- When using the Hidden Needle Cover with HumatroPen 12 mg, repeat steps 1 to 10 of the Hidden Needle Cover Instructions for Use.
Commonly asked questions:
-
Do I need to perform the New Cartridge Setup before every dose?
- No. You only need to perform the New Cartridge Set-up 1 time for each Cartridge, just before a new Cartridge is used for the first time.
- The purpose of the New Cartridge Set-up is to make sure the HumatroPen 12 mg with an attached HUMATROPE 12 mg Cartridge are ready to use.
- If you repeat the New Cartridge Set-up before each routine dose, you may run out of HUMATROPE early. The small amount of medicine used in the New Cartridge Set-up will not affect the amount of HUMATROPE in the Cartridge.
-
What should I do if the Cartridge label and Pen do not match?
- Do not use the Pen if the Cartridge strength on the HUMATROPE Cartridge label does not match the number on the Pen's Front Housing. This is important to make sure the correct dose of HUMATROPE is given.
- Contact your healthcare provider for a replacement.
-
What should I do if the HUMATROPE is not clear?
- Do not use the Pen if the liquid is cloudy or contains particles. Contact your healthcare provider or call Lilly at 1-800-545-5979 or go to www.humatrope.com.
-
Why are there air bubbles in the Cartridge?
- Air bubbles may remain in the Cartridge after mixing.
- If the Pen is stored with a Needle attached, air bubbles may form in the Cartridge.
Do not store the Pen with a Needle attached. - Follow the instructions in Section 2, Step 2D (Remove air from new Cartridge) to remove air bubbles from the Cartridge.
- It is normal to see small air bubbles. It will not cause any harm or affect the dose.
-
What should I do if the screw does not move out when there is no Cartridge attached to the Pen?
- The screw may not move out when you push the Injection Button unless there is a Cartridge in the Pen. This allows you to easily push the screw into the Pen Body when replacing a Cartridge.
- After a Cartridge is attached, the screw will move out when the Injection Button is pushed.
-
What should I do if I cannot attach the Cartridge to the Pen Body?
- Check that the Cartridge is not damaged or broken.
- Carefully line up the Cartridge with the Pen Body and screw them together until secure. If the Cartridge and Pen cannot be screwed together, contact your healthcare provider.
-
Why is it hard to push the Injection Button when I try to inject the dose?
- The Needle may be clogged. Try attaching a new Needle.
- Pushing the Injection Button down quickly may make the Injection Button harder to push. Pushing the Injection Button more slowly may make it easier.
- Using a larger Needle will make it easier to push the Injection Button during injection. Ask your healthcare provider which Needle is best for you.
- The Injection Button may become harder to push if the inside of the Pen gets dirty with HUMATROPE, food, drink, or other materials.
-
What should I do if the dose knob does not go to zero (0) when I inject the dose?
- This can happen if the HUMATROPE Cartridge does not have enough HUMATROPE left in it for the full dose.
- At the end of the injection, the number in the dose window should be 0.00. If you see any number other than “0.00”, this is the amount of HUMATROPE you did not receive. Write this number down and follow the steps below to get the rest of your dose.
- To get the rest of your dose:
- -
- Remove the Needle and empty Cartridge from the Pen.
- -
- Insert a new Cartridge, attach a new Needle, and remove air from the new Cartridge (see Steps 2A through 2D).
- -
- Complete your dose by injecting only the amount you did not receive (see Step 3C).
- -
- If you still do not think you received your full dose, do not take another dose. Call Lilly at 1-800-545-5979 or your healthcare provider for assistance.
-
What should I do if I see HUMATROPE leaking from the Needle after I have finished the injection?
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
- -
- The full dose may not have been delivered. Do not inject another dose. Talk with your healthcare provider for assistance.
- -
- To prevent this, for the next dose, firmly push and hold the Injection Button in and slowly count to 5 (see Section 3, Step 3C).
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
-
How can I tell when the injection is complete?
- The injection is complete when:
- -
- You place your thumb on the injection Button, then slowly and firmly push the Injection Button until it stops moving.
- -
- Continue to hold the Injection Button for 5 seconds, then remove the Needle from the skin.
- -
- You will see 0.00 in the center of the dose window.
- The injection is complete when:
If you have any questions or problems with your HumatroPen 12 mg, contact Lilly at 1-800-545-5979 or your healthcare professional for assistance.
Report all MEDICAL DEVICE COMPLAINTS or UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to Eli Lilly and Company, by contacting them at 1-800-545-5979.
You are also encouraged to report all UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to the U.S. FOOD & DRUG Administration (US FDA) by:
- Visiting the MedWatch web page on reporting an adverse event or serious problem to FDA at (https://www.fda.gov/safety/reporting-serious-problems-fda/how-consumers-can-report-adverse-event-or-serious-problem-fda) or
- Calling FDA’s toll-free information line at 1-800-INFO-FDA (1-888-463-6332).
Disposing of used Needles:
- Put your used Needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose Needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles. Do not reuse or share your Needles with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

Eli Lilly and Company
Pharmaceutical Delivery Systems
Lilly Corporate Center
Indianapolis, IN 46285, USA
US License Number 1891
Eli Lilly Nederland B.V.
Papendorpseweg 83
3528 BJ UTRECHT
The NetherlandsHUMATROPE® and HumatroPen® 12 mg are registered trademarks of Eli Lilly and Company.
The HumatroPen® 12 mg meets the current dose accuracy and functional requirements of ISO 11608-1 and is for use with HUMATROPE® 12 mg Cartridges only.
Copyright © 2009, 2023, Eli Lilly and Company. All rights reserved.
Revised December 2023Non-harmonized Symbol Glossary1
1 Glossary includes non-harmonized symbols used in the labeling.
2 Symbol used only on the carton.HTRPEN12-0005-IFU-20231208
Close -
Humatrope 24 mg HumatroPen Instuctions for UseInstructions for Use - HumatroPen® 24 mg - (HU-ma-tro-Pen 24 mg) HUMATROPE® Delivery System Device - for use with - HUMATROPE® (somatropin) 24 mg Cartridges - 24 mg - Section 1 ...
Instructions for Use
HumatroPen® 24 mg
(HU-ma-tro-Pen 24 mg)
HUMATROPE® Delivery System Device
for use with
HUMATROPE® (somatropin) 24 mg Cartridges
Section 1: Read Section 1 completely before you move on to Section 2.
What you need to know about HumatroPen® 24 mg
Read this Instructions for Use including the “Commonly asked questions” section, at the end of this Instructions for Use, before you start using HumatroPen 24 mg and each time you get a new Pen. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Your healthcare provider should show you how to use HumatroPen (Pen) the right way before you use it for the first time. Ask your healthcare provider if you have any questions.
 If you are having problems using the HumatroPen 24 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
If you are having problems using the HumatroPen 24 mg, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Introduction
Intended Use:
- The HumatroPen 24 mg is a reusable pen-injector designed for self-administration of human growth hormone. The pen-injector is intended for use with the specified HUMATROPE 24 mg Cartridges and single use, detachable and disposable Pen Needles (supplied separately). Your healthcare provider will prescribe the HUMATROPE dose and Pen that is right for you or your child. Do not change your dose or use another type of pen unless your healthcare provider tells you to.
- If your healthcare provider changes the prescribed cartridge strength from the 24 mg HUMATROPE Cartridge to the 6 mg or 12 mg HUMATROPE Cartridge, you will need to get a new HumatroPen to match the new cartridge strength.
Contraindications:
There are no known contraindications for HumatroPen 24 mg.
Important information about HumatroPen 24 mg:
- Do not use the Pen if any part of the Pen or Cartridge looks broken or damaged. Contact your healthcare provider.
- Make sure that you have a 24 mg HUMATROPE Cartridge to match the HumatroPen 24 mg. If the strength on the label for the Cartridge and the Pen do not match, do not use them and contact your healthcare provider. It is important to use the correct Pen and Cartridge to make sure that you inject the correct dose of HUMATROPE.
- Do not use the HUMATROPE Cartridge past the expiration date.
- Follow the instructions in Section 2 only to set up a new Cartridge before you use it for the first time.
- Follow the instructions in Section 3 of this Instructions for Use for every injection.
- Do not transfer the contents of the HUMATROPE Cartridge to a syringe.
- Do not share your HumatroPen 24 mg or Pen Needles with anyone else, even if the Needle has been changed. You may give other people a serious infection or get a serious infection from them.
- The HumatroPen 24 mg is not recommended for use by blind or visually impaired individuals without the help of a sighted individual trained on how to use it.
Information about Needles used with HumatroPen 24 mg:
- Pen Needles are not included with HumatroPen. You may need a prescription to get the Needles from your pharmacist.
- Becton, Dickinson and Company Pen Needles are recommended for use with the HumatroPen 24 mg.
- Ask your healthcare provider what size Needle is best for you to use.
- Use of the Hidden Needle Cover with your HumatroPen is optional.
- When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
- Follow your healthcare provider's instructions about how to safely handle Needles.
- Always remove the Pen Needle after each injection and use a new Needle with each injection. This helps reduce the risk of infection, prevent leakage of HUMATROPE, reduce air bubbles, and reduce Needle clogs.
- For information on the right way to throw away (dispose of) used Needles see “Disposing of used Needles” at the end of this Instructions for Use.
How do I care for HumatroPen 24 mg?
- Soiled parts including the Hidden Needle Cover can be cleaned with a damp cloth. Do not use alcohol or any other cleaning product.
- Keep dry.
- Do not apply oil or any other lubricant.
How do I store HumatroPen 24 mg with an attached cartridge?
- Store HumatroPen 24 mg between -40°C (-40°F) to 70°C (158°F) without the Humatrope 24 mg Cartridge.
- After attaching a prepared HUMATROPE cartridge to your HumatroPen 24 mg, store it in the storage case in the refrigerator between 36°F to 46°F (+2°C to +8°C) until it is time for your next injection.
- Before injecting a dose of HUMATROPE, take the Pen out of the refrigerator and let it sit for 10 minutes to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort. Do not leave the HumatroPen 24 mg and HUMATROPE cartridge out of the refrigerator for more than 30 minutes a day.
- HUMATROPE Cartridges attached to a Pen can be stored in the refrigerator for up to 28 days. Do not use a prepared Cartridge after 28 days.
- Do not freeze.
- Do not store the Pen with the Needle attached.
- Protect HUMATROPE Cartridges attached to the Pen from light during storage.
When do I need to replace and dispose my HumatroPen?
Do not use your pen for more than 3 years after the first use or past the Use-by date on the carton, whichever comes first.
The expired Pen may be discarded in your household trash after you have removed the Needle.
Record the date the Pen was first used here: __ / __ / __.
Record the Use-by date from your carton here: __ / __ / __.Contact your healthcare provider if you need a new HumatroPen 24 mg, or when the Pen has been used for 3 years.
 Please see the accompanying complete Humatrope Patient Information Sheet.
Please see the accompanying complete Humatrope Patient Information Sheet.
For more information, call Lilly at 1-800-545-5979 or go to www.humatrope.com.
Section 2: Read Section 2 only after you have read Section 1.
Getting Started
- Be sure to follow the reconstitution (mixing) directions in the HUMATROPE Cartridge Kit.
- Perform the New Cartridge Set-up only 1 time at the beginning of each new Cartridge preparation.
- For daily use, do not repeat the 1 time only New Cartridge Set-up. If you repeat the 1 time only New Cartridge Set-up, you may run out of HUMATROPE early.
New Cartridge Set-up
Important Notes:
- The Pen must be set-up before injecting the first dose from each new 24 mg Cartridge.
- Setting up the new Cartridge is important to remove large air bubbles that may be present after mixing the HUMATROPE 24 mg Cartridge.
- If liquid is not seen at the Needle tip after you have tried several times to remove air from the new Cartridge as instructed in Step 2D, contact your healthcare provider or Lilly at 1-800-545-5979.
Step 2E – Continue on to Daily Use
- Do not repeat the New Cartridge Set-up before each dose.
- Leave the Cartridge attached and do not remove it until the Cartridge is empty.
- If you are ready to inject the first dose and you have finished setting up your Pen with a new Cartridge, go to Section 3, Step 3C.
- If you are using the Hidden Needle Cover with HumatroPen 24 mg, read the Hidden Needle Cover Instructions for Use.
Section 3: Now that you have done the 1 time only New Cartridge Set-up, follow Section 3 for each of your daily injections.
- Remove the prepared Pen with the Cartridge attached from your refrigerator.
- Let the Pen with Cartridge attached stand at room temperature for 10 minutes before injecting.
Daily Use Before your injection: Take the Pen out of the refrigerator and let it sit for 10 minutes before injecting to allow it to reach room temperature. Injecting medicine that is cold can cause discomfort.
Step 3A – Check the PenStep 3B – Attach a new disposable Pen Needle for each injection.
- Remove the paper tab from the end of the Outer Cap.
- Push the Needle straight onto the 24 mg Cartridge and turn it until secure.
- Pull off the Outer Cap and the Inner Cap.
- Keep the Outer Cap to remove the Needle after the injection.
Important Note: Talk to your healthcare provider about the right way to use the Pen, and the optional Hidden Needle Cover, the right sites to inject HUMATROPE, and how to rotate your injection sites. When using the Hidden Needle Cover with your HumatroPen, you must use a Needle length of 5 mm or longer. Read the Hidden Needle Cover Instructions for Use.
Step 3C – Dial and inject the dose
Step 3D – Remove and dispose of the Needle
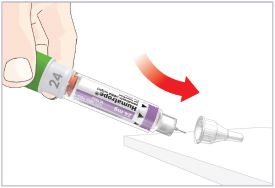
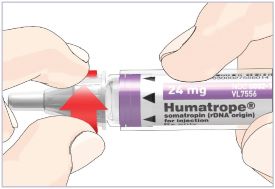
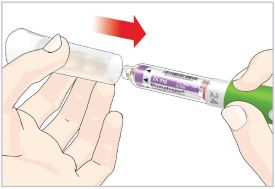
- Remove the capped Needle. Follow the instructions in the “Disposing of used Needles” section at the end of this Instructions for Use to dispose of the Needle.
- Replace the Pen Cap.
Important Note: Do not remove this Cartridge from the Pen until the Cartridge is empty or needs to be replaced to help prevent you from giving the wrong dose.
Step 3E – Store Pen and Cartridge for next use
- See, “How do I store HumatroPen 24 mg?” in Section 1 of this Instructions for Use for more information.
- When it is time for the next scheduled dose, go to Section 3, and repeat Steps 3A through 3D.
- When using the Hidden Needle Cover with HumatroPen 24 mg, repeat steps 1 to 10 of the Hidden Needle Cover Instructions for Use.
Commonly asked questions:
-
Do I need to perform the New Cartridge Setup before every dose?
- No. You only need to perform the New Cartridge Set-up 1 time for each Cartridge, just before a new Cartridge is used for the first time.
- The purpose of the New Cartridge Set-up is to make sure the HumatroPen 24 mg with an attached HUMATROPE 24 mg Cartridge are ready to use.
- If you repeat the New Cartridge Set-up before each routine dose, you may run out of HUMATROPE early. The small amount of medicine used in the New Cartridge Set-up will not affect the amount of HUMATROPE in the Cartridge.
-
What should I do if the Cartridge label and Pen do not match?
- Do not use the Pen if the Cartridge strength on the HUMATROPE Cartridge label does not match the number on the Pen's Front Housing. This is important to make sure the correct dose of HUMATROPE is given.
- Contact your healthcare provider for a replacement.
-
What should I do if the HUMATROPE is not clear?
- Do not use the Pen if the liquid is cloudy or contains particles. Contact your healthcare provider or call Lilly at 1-800-545-5979 or go to www.humatrope.com.
-
Why are there air bubbles in the Cartridge?
- Air bubbles may remain in the Cartridge after mixing.
- If the Pen is stored with a Needle attached, air bubbles may form in the Cartridge.
Do not store the Pen with a Needle attached. - Follow the instructions in Section 2, Step 2D (Remove air from new Cartridge) to remove air bubbles from the Cartridge.
- It is normal to see small air bubbles. It will not cause any harm or affect the dose.
-
What should I do if the screw does not move out when there is no Cartridge attached to the Pen?
- The screw may not move out when you push the Injection Button unless there is a Cartridge in the Pen. This allows you to easily push the screw into the Pen body when replacing a Cartridge.
- After a Cartridge is attached, the screw will move out when the Injection Button is pushed.
-
What should I do if I cannot attach the Cartridge to the Pen Body?
- Check that the Cartridge is not damaged or broken.
- Carefully line up the Cartridge with the Pen body and screw them together until secure. If the Cartridge and Pen cannot be screwed together, contact your healthcare provider.
-
Why is it hard to push the Injection Button when I try to inject the dose?
- The Needle may be clogged. Try attaching a new Needle.
- Pushing the Injection Button down quickly may make the Injection Button harder to push. Pushing the Injection Button more slowly may make it easier.
- Using a larger Needle will make it easier to push the Injection Button during injection. Ask your healthcare provider which Needle is best for you.
- The Injection Button may become harder to push if the inside of the Pen gets dirty with HUMATROPE, food, drink, or other materials.
-
What should I do if the dose knob does not go to zero (0) when I inject the dose?
- This can happen if the HUMATROPE Cartridge does not have enough HUMATROPE left in it for the full dose.
- At the end of the injection, the number in the dose window should be 0.00. If you see any number other than “0.00”, this is the amount of HUMATROPE you did not receive. Write this number down and follow the steps below to get the rest of your dose.
- To get the rest of your dose:
- -
- Remove the Needle and empty Cartridge from the Pen.
- -
- Insert a new Cartridge, attach a new Needle, and remove air from the new Cartridge (see Steps 2A through 2D).
- -
- Complete your dose by injecting only the amount you did not receive (see Step 3C).
- -
- If you still do not think you received your full dose, do not take another dose. Call Lilly at 1-800-545-5979 or your healthcare provider for assistance.
-
What should I do if I see HUMATROPE leaking from the Needle after I have finished the injection?
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
- -
- The full dose may not have been delivered. Do not inject another dose. Talk with your healthcare provider for assistance.
- -
- To prevent this, for the next dose, firmly push and hold the Injection Button in and slowly count to 5 (see Section 3, Step 3C).
- It is normal for a single drop to remain on the tip of the Needle after the injection is complete. If you see more than 1 drop:
-
How can I tell when the injection is complete?
- The injection is complete when:
- -
- You place your thumb on the Injection Button, then slowly and firmly push the Injection Button until it stops moving.
- -
- Continue to hold the Injection Button for 5 seconds, then remove the Needle from the skin.
- -
- You will see 0.00 in the center of the dose window.
- The injection is complete when:
If you have any questions or problems with your HumatroPen 24 mg, contact Lilly at 1-800-545-5979 or your healthcare professional for assistance.
Report all MEDICAL DEVICE COMPLAINTS or UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to Eli Lilly and Company, by contacting them at 1-800-545-5979.
You are also encouraged to report all UNDESIRABLE SIDE EFFECTS, including SUSPECTED SERIOUS INCIDENTS, to the U.S. FOOD & DRUG Administration (US FDA) by:
- Visiting the MedWatch web page on reporting an adverse event or serious problem to FDA at (https://www.fda.gov/safety/reporting-serious-problems-fda/how-consumers-can-report-adverse-event-or-serious-problem-fda) or
- Calling FDA’s toll-free information line at 1-800-INFO-FDA (1-888-463-6332).
Disposing of used Needles:
- Put your used Needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose Needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles. Do not reuse or share your Needles with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

Eli Lilly and Company
Pharmaceutical Delivery Systems
Lilly Corporate Center
Indianapolis, IN 46285, USA
US License Number 1891
Eli Lilly Nederland B.V.
Papendorpseweg 83
3528 BJ UTRECHT
The NetherlandsHUMATROPE® and HumatroPen® 24 mg are registered trademarks of Eli Lilly and Company.
The HumatroPen® 24 mg meets the current dose accuracy and functional requirements of ISO 11608-1 and is for use with HUMATROPE® 24 mg Cartridges only.
Copyright © 2009, 2023, Eli Lilly and Company. All rights reserved.
Revised December 2023Non-harmonized Symbol Glossary1
1 Glossary includes non-harmonized symbols used in the labeling.
2 Symbol used only on the carton.HTRPEN24-0005-IFU-20231208
Close -
Humatrope Hidden Needle Cover for use with HumatroPen Instuctions for UseInstructions for Use - Hidden Needle Cover - For use with HumatroPen® (6 mg, 12 mg, or 24 mg) What you need to know about the Hidden Needle Cover - Read this Instructions for Use ...
Instructions for Use
Hidden Needle Cover
For use with HumatroPen® (6 mg, 12 mg, or 24 mg)
What you need to know about the Hidden Needle Cover
Read this Instructions for Use for the Hidden Needle Cover before you use it. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Your healthcare provider should show you how to use the Hidden Needle Cover with the HumatroPen the right way before you use it for the first time. Ask your healthcare provider if you have any questions. If you are having problems using the Hidden Needle Cover with the HumatroPen (6 mg, 12 mg or 24 mg) call Lilly at 1-800-545-5979 or go to www. Humatrope.com.
The Hidden Needle Cover allows you to give your injection with the needle hidden from view. Be sure the HumatroPen is set up by following the steps in the HumatroPen Instructions for Use.
What kinds of needles can be used with the Hidden Needle Cover?
- Pen needles are not included. You may need a prescription from your healthcare provider to get the needles from your pharmacist.
- Becton, Dickinson and Company pen needles are recommended for use with the HumatroPen.
- Use of the Hidden Needle Cover with your HumatroPen is optional.
- When using the Hidden Needle Cover with your HumatroPen, you must use a needle length of 5 mm or longer.
- Ask your healthcare provider which needle is best for you.
1 Be sure the HumatroPen cap is removed. Remove the paper tab and attach a new needle to the Cartridge, and remove the outer needle cap and inner needle cap. Read Section 2A and 2B of the HumatroPen Instructions for Use for detailed instructions to set-up the HumatroPen. Read Section 2C of the HumatroPen Instructions for Use for detailed instructions to attach the needle. Follow your healthcare provider's instructions on the safe handling of needles. 9 Read Step 3D “Remove and dispose of the needle” in the Instructions for Use for the HumatroPen.
10 Read Step 3E “Store Pen and Cartridge for next use” in the Instructions for Use for the HumatroPen.
11 Read “Commonly asked questions” in the Instructions for Use for the HumatroPen.
12 Read “Disposing of used needles” in the Instructions for Use for the HumatroPen.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

Eli Lilly and Company
Pharmaceutical Delivery Systems
Lilly Corporate Center
Indianapolis, IN 46285, USA
US License Number 1891HUMATROPE® and HumatroPen® are registered trademarks of Eli Lilly and Company.
Copyright © 2009, 2023, Eli Lilly and Company. All rights reserved.
Revised December 2023HTRHNC-0003-IFU-20231208
Close -
PACKAGE LABEL – Humatrope 6 mg Cartridge Kit
6 mg - Combination Package - NDC 0002-8147-01 - MS 8147 - Humatrope® (somatropin) for injection - 6 mg - Cartridge Kit - Single-Patient-Use - for use only with the Humatrope® (somatropin) for injection ...
6 mg
Combination Package
NDC 0002-8147-01
MS 8147
Humatrope®
(somatropin) for injection
6 mg
Cartridge Kit
Single-Patient-Use
for use only with the Humatrope®
(somatropin) for injectionpen injection device
Rx only
Refrigerate
Do Not Freeze
Do Not Shake
Kit contains:
One Humatrope Cartridge 6 mg
One Prefilled Diluent Syringe
www.humatrope.com
Lilly
Close -
PACKAGE LABEL – Humatrope 12 mg Cartridge Kit
12 mg - Combination Package - NDC 0002-8148-01 - MS 8148 - Humatrope® (somatropin) for injection - 12 mg - Cartridge Kit - Single-Patient-Use - for use only with the Humatrope® (somatropin) for ...
12 mg
Combination Package
NDC 0002-8148-01
MS 8148
Humatrope®
(somatropin) for injection
12 mg
Cartridge Kit
Single-Patient-Use
for use only with the Humatrope®
(somatropin) for injectionpen injection device
Rx only
Refrigerate
Do Not Freeze
Do Not Shake
Kit contains:
One Humatrope Cartridge 12 mg
One Prefilled Diluent Syringe
www.humatrope.com
Lilly
Close -
PACKAGE LABEL – Humatrope 24 mg Cartridge Kit
24 mg - Combination Package - NDC 0002-8149-01 - MS 8149 - Humatrope® (somatropin) for injection - 24 mg - Cartridge Kit - Single-Patient-Use - for use only with the Humatrope® (somatropin) for injection ...
24 mg
Combination Package
NDC 0002-8149-01
MS 8149
Humatrope®
(somatropin) for injection24 mg
Cartridge Kit
Single-Patient-Use
for use only with the Humatrope®
(somatropin) for injectionpen injection device
Rx only
Refrigerate
Do Not Freeze
Do Not Shake
Kit contains:
One Humatrope Cartridge 24 mg
One Prefilled Diluent Syringe
www.humatrope.com
Lilly
Close -
PACKAGE LABEL – Humatrope 6 mg Pen
Humatrope® (somatropin) for injection - HumatroPen® 6 mg - Growth Hormone Delivery System - Injection Device for Use with Humatrope® (somatropin) 6 mg Cartridges - HumatroPen® 6 mg is recommended for ...
Humatrope®
(somatropin) for injection
HumatroPen® 6 mg
Growth Hormone Delivery System
Injection Device for Use with Humatrope® (somatropin) 6 mg Cartridges
HumatroPen® 6 mg is recommended for use with Becton, Dickinson and Company pen needles
Not included: Humatrope® cartridge and Becton, Dickinson and Company pen needles.
Contents
HumatroPen® 6 mg
Pen Case
HumatroPen® 6 mg User Manual
Hidden Needle Cover
Hidden Needle Cover User Manual
MS9560
Lilly
Close -
PACKAGE LABEL – Humatrope 12 mg Pen
Humatrope® (somatropin) for injection - HumatroPen® 12 mg - Growth Hormone Delivery System - Injection Device for Use with Humatrope® (somatropin) 12 mg Cartridges - HumatroPen® 12 mg is recommended ...
Humatrope®
(somatropin) for injection
HumatroPen® 12 mg
Growth Hormone Delivery System
Injection Device for Use with Humatrope® (somatropin) 12 mg Cartridges
HumatroPen® 12 mg is recommended for use with Becton, Dickinson and Company pen needles
Not included: Humatrope® cartridge and Becton, Dickinson and Company pen needles.
Contents
HumatroPen® 12 mg
Pen Case
HumatroPen® 12 mg User Manual
Hidden Needle Cover
Hidden Needle Cover User Manual
MS9561
Lilly
Close -
PACKAGE LABEL – Humatrope 24 mg Pen
Humatrope® (somatropin) for injection - HumatroPen® 24 mg - Growth Hormone Delivery System - Injection Device for Use with Humatrope® (somatropin) 24 mg Cartridges - HumatroPen® 24 mg is recommended ...
Humatrope®
(somatropin) for injection
HumatroPen® 24 mg
Growth Hormone Delivery System
Injection Device for Use with Humatrope® (somatropin) 24 mg Cartridges
HumatroPen® 24 mg is recommended for use with Becton, Dickinson and Company pen needles
Not included: Humatrope® cartridge and Becton, Dickinson and Company pen needles.
Contents
HumatroPen® 24 mg
Pen Case
HumatroPen® 24 mg User Manual
Hidden Needle Cover
Hidden Needle Cover User Manual
MS9562
Lilly
Close -
INGREDIENTS AND APPEARANCEProduct Information
HUMATROPE somatropin kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-8147 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-8147-01 1 in 1 CARTON 01/27/2006 1 1 in 1 TRAY; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CARTRIDGE 2.88 mL Part 2 1 SYRINGE 2.88 mL Part 1 of 2 HUMATROPE somatropin injection, powder, for solution Product Information Item Code (Source) NDC:0002-7554 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Somatropin (UNII: NQX9KB6PCL) (Somatropin - UNII:NQX9KB6PCL) Somatropin 6 mg in 2.88 mL Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) 18 mg in 2.88 mL glycine (UNII: TE7660XO1C) 6 mg in 2.88 mL sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) 1.36 mg in 2.88 mL sodium hydroxide (UNII: 55X04QC32I) phosphoric acid (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7554-01 2.88 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Part 2 of 2 STERILE DILUENT diluent injection, solution Product Information Item Code (Source) NDC:0002-7618 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Metacresol (UNII: GGO4Y809LO) 9.9 mg in 2.88 mL glycerin (UNII: PDC6A3C0OX) 53.2 mg in 2.88 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7618-01 2.88 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 HUMATROPE somatropin kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-8148 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-8148-01 1 in 1 CARTON 01/27/2006 1 1 in 1 TRAY; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CARTRIDGE 2.88 mL Part 2 1 SYRINGE 2.88 mL Part 1 of 2 HUMATROPE somatropin injection, powder, for solution Product Information Item Code (Source) NDC:0002-7555 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Somatropin (UNII: NQX9KB6PCL) (Somatropin - UNII:NQX9KB6PCL) Somatropin 12 mg in 2.88 mL Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) 36 mg in 2.88 mL glycine (UNII: TE7660XO1C) 12 mg in 2.88 mL sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) 2.72 mg in 2.88 mL sodium hydroxide (UNII: 55X04QC32I) phosphoric acid (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7555-01 2.88 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Part 2 of 2 STERILE DILUENT diluent injection, solution Product Information Item Code (Source) NDC:0002-7619 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Metacresol (UNII: GGO4Y809LO) 9.8 mg in 2.88 mL glycerin (UNII: PDC6A3C0OX) 9.0 mg in 2.88 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7619-01 2.88 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 HUMATROPE somatropin kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-8149 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-8149-01 1 in 1 CARTON 01/27/2006 1 1 in 1 TRAY; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CARTRIDGE 2.88 mL Part 2 1 SYRINGE 2.88 mL Part 1 of 2 HUMATROPE somatropin injection, powder, for solution Product Information Item Code (Source) NDC:0002-7556 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Somatropin (UNII: NQX9KB6PCL) (Somatropin - UNII:NQX9KB6PCL) Somatropin 24 mg in 2.88 mL Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) 72 mg in 2.88 mL glycine (UNII: TE7660XO1C) 24 mg in 2.88 mL sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) 5.43 mg in 2.88 mL sodium hydroxide (UNII: 55X04QC32I) phosphoric acid (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7556-01 2.88 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Part 2 of 2 STERILE DILUENT diluent injection, solution Product Information Item Code (Source) NDC:0002-7619 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Metacresol (UNII: GGO4Y809LO) 9.8 mg in 2.88 mL glycerin (UNII: PDC6A3C0OX) 9.0 mg in 2.88 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-7619-01 2.88 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019640 01/27/2006
CloseLabeler - Eli Lilly and Company (006421325)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
HUMATROPE- somatropin kit
Number of versions: 29
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 12, 2024 | 41 (current) | download |
| Dec 6, 2024 | 39 | download |
| Dec 22, 2023 | 38 | download |
| Jun 28, 2023 | 37 | download |
| Dec 19, 2022 | 36 | download |
| Aug 13, 2021 | 35 | download |
| Mar 25, 2021 | 34 | download |
| Sep 3, 2020 | 32 | download |
| Nov 12, 2019 | 31 | download |
| Sep 6, 2019 | 30 | download |
| Nov 21, 2017 | 29 | download |
| May 15, 2017 | 28 | download |
| Dec 20, 2016 | 26 | download |
| Sep 30, 2016 | 25 | download |
| Apr 20, 2015 | 24 | download |
| Aug 12, 2014 | 23 | download |
| Apr 29, 2013 | 21 | download |
| Sep 11, 2012 | 19 | download |
| Apr 25, 2012 | 18 | download |
| Dec 15, 2011 | 17 | download |
| Oct 17, 2011 | 16 | download |
| Apr 18, 2011 | 15 | download |
| Mar 28, 2011 | 14 | download |
| Mar 8, 2011 | 13 | download |
| Jan 20, 2011 | 12 | download |
| Oct 15, 2010 | 11 | download |
| Aug 11, 2010 | 9 | download |
| Oct 9, 2009 | 7 | download |
| Sep 6, 2007 | 1 | download |
RxNorm
HUMATROPE- somatropin kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 582969 | somatropin 12 MG Cartridge | PSN |
| 2 | 582969 | somatropin 12 MG Cartridge | SCD |
| 3 | 582971 | Humatrope 12 MG Cartridge | PSN |
| 4 | 582971 | somatropin 12 MG Cartridge [Humatrope] | SBD |
| 5 | 582971 | Humatrope 12 MG Cartridge | SY |
| 6 | 582974 | somatropin 24 MG Cartridge | PSN |
| 7 | 582974 | somatropin 24 MG Cartridge | SCD |
| 8 | 582976 | Humatrope 24 MG Cartridge | PSN |
| 9 | 582976 | somatropin 24 MG Cartridge [Humatrope] | SBD |
| 10 | 582976 | Humatrope 24 MG Cartridge | SY |
| 11 | 729232 | somatropin 6 MG Cartridge | PSN |
| 12 | 729232 | somatropin 6 MG Cartridge | SCD |
| 13 | 729234 | Humatrope 6 MG Cartridge | PSN |
| 14 | 729234 | somatropin 6 MG Cartridge [Humatrope] | SBD |
| 15 | 729234 | Humatrope 6 MG Cartridge | SY |
Get Label RSS Feed for this Drug
HUMATROPE- somatropin kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=a774e1ae-3997-49ee-8b0e-99a2b315d409
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
HUMATROPE- somatropin kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0002-7554-01 |
| 2 | 0002-7555-01 |
| 3 | 0002-7556-01 |
| 4 | 0002-7618-01 |
| 5 | 0002-7619-01 |
| 6 | 0002-8147-01 |
| 7 | 0002-8148-01 |
| 8 | 0002-8149-01 |











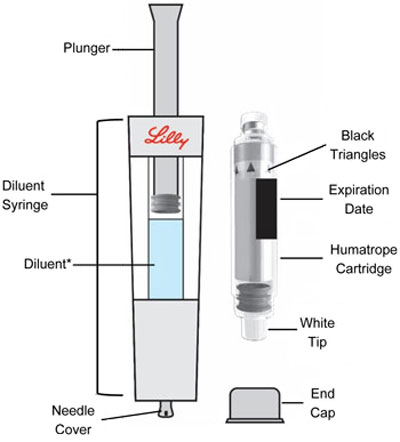
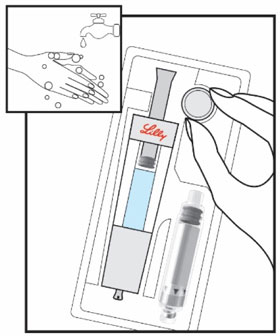
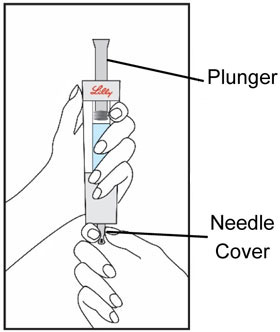
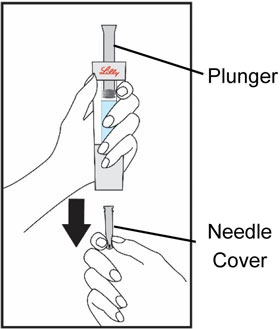
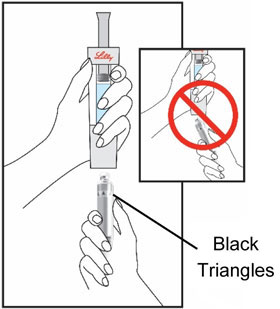
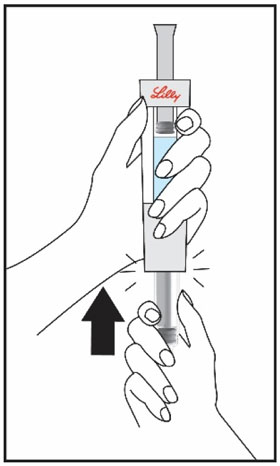
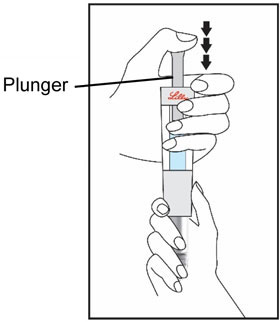
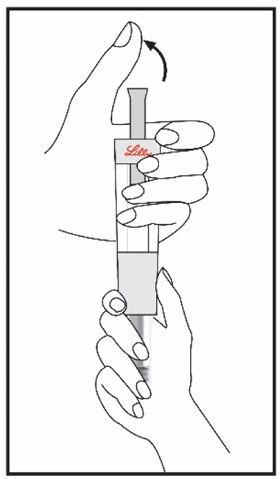
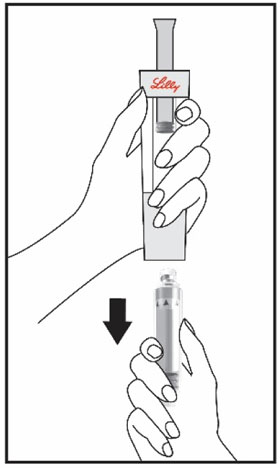
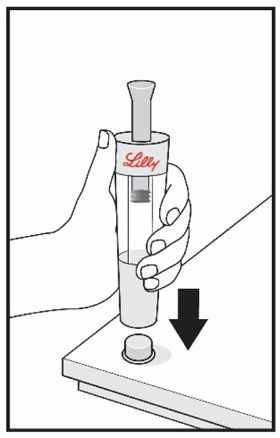
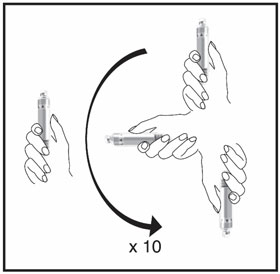



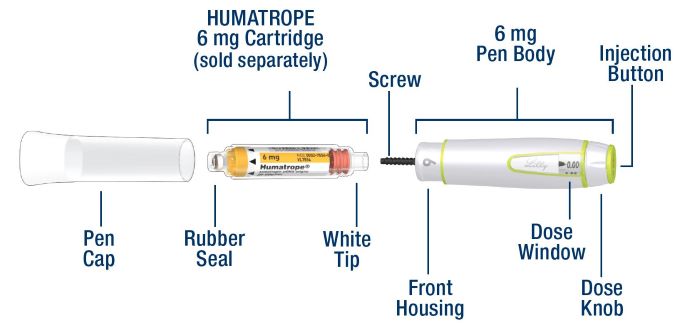
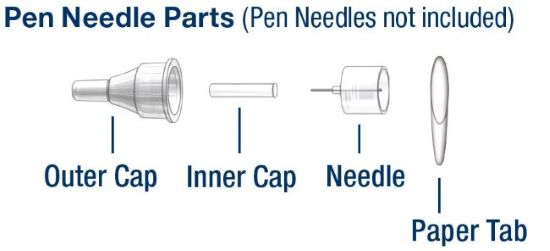

 Front View
Front View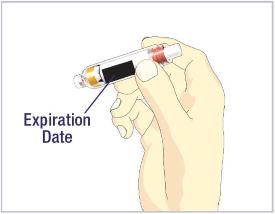 Back View
Back View
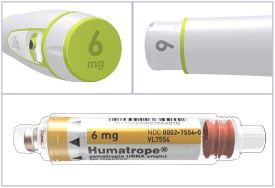
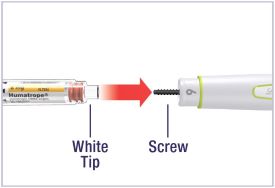
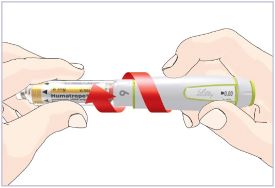
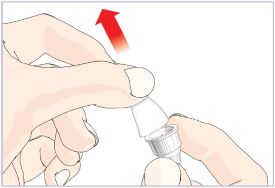
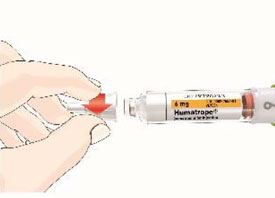
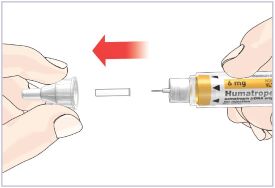
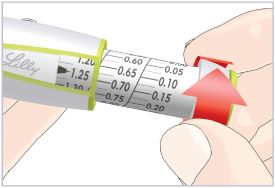
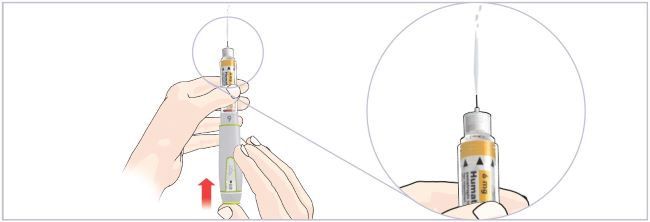
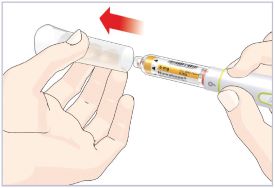
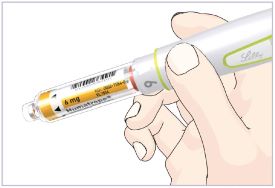
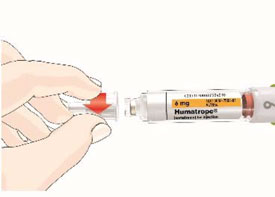
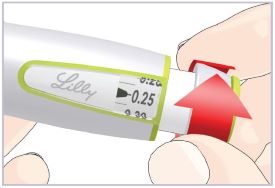
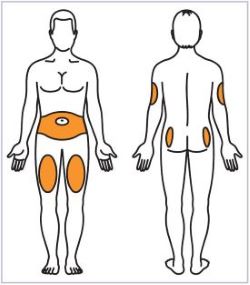
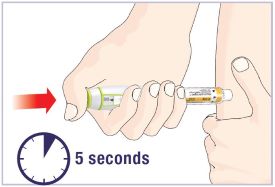
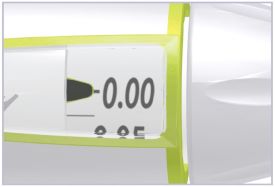
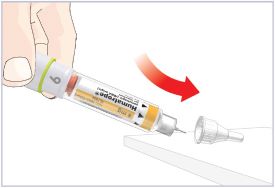
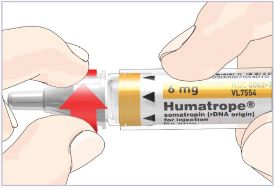
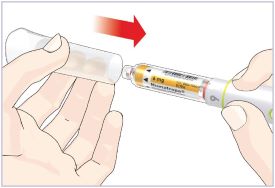


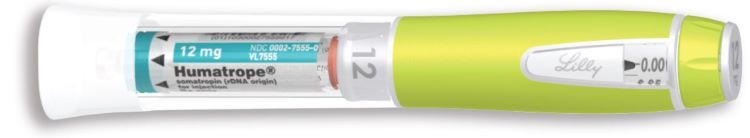
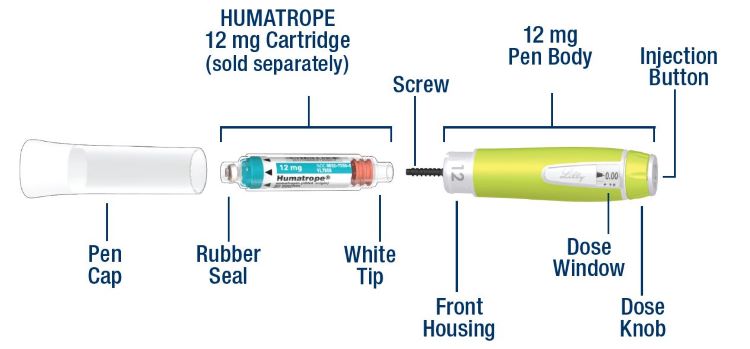
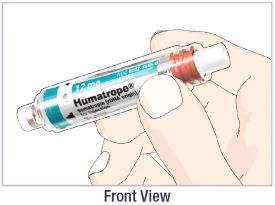 Front View
Front View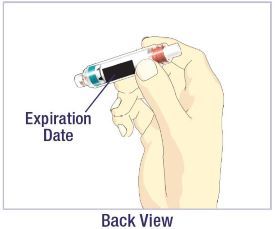 Back View
Back View
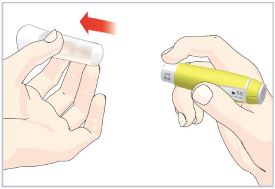
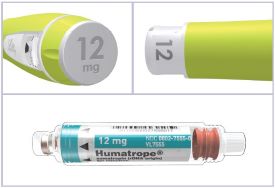
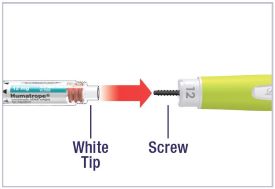
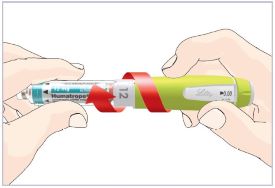
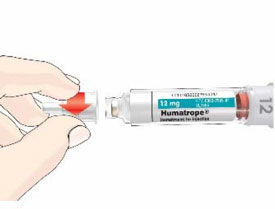
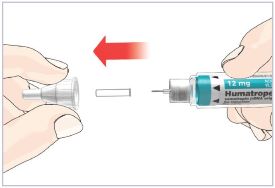
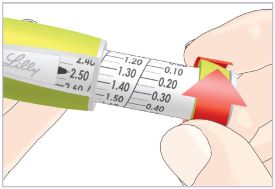
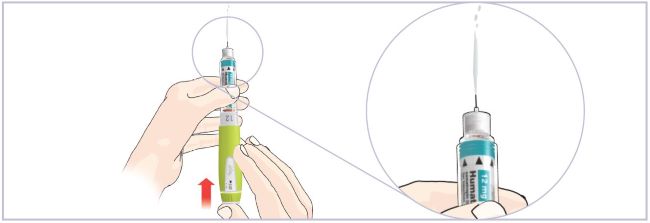
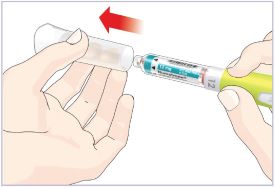
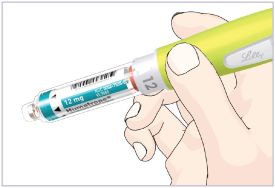
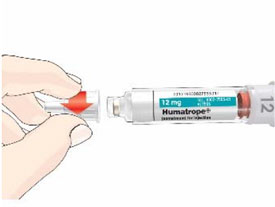
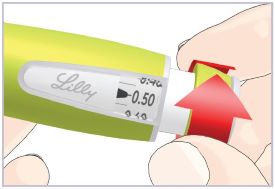
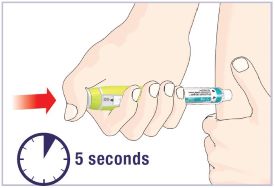

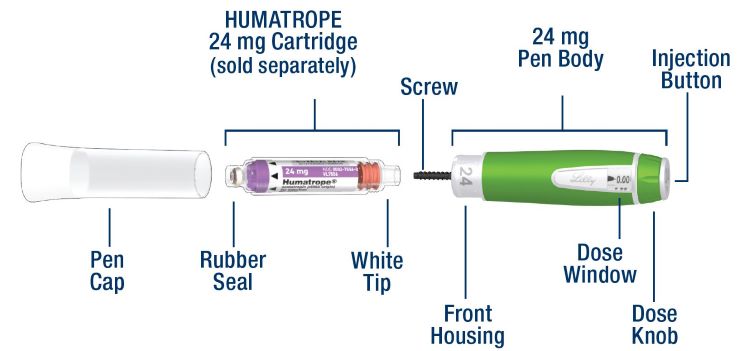
 Front View
Front View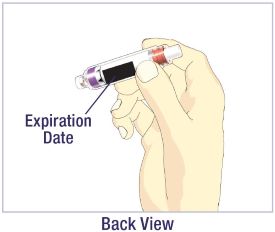 Back View
Back View