Label: CYSVIEW- hexaminolevulinate hydrochloride kit
- NDC Code(s): 10511-3001-2
- Packager: Photocure Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Cysview safely and effectively. See full prescribing information for Cysview. Cysview (hexaminolevulinate hydrochloride) ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECysview is indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a ...
-
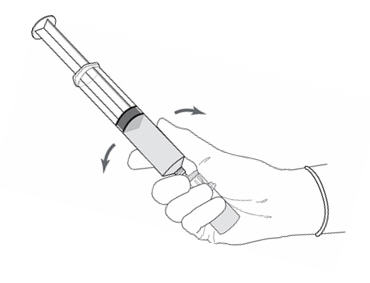
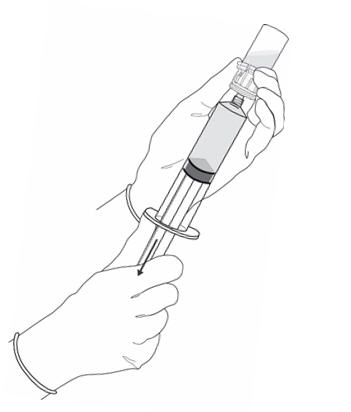
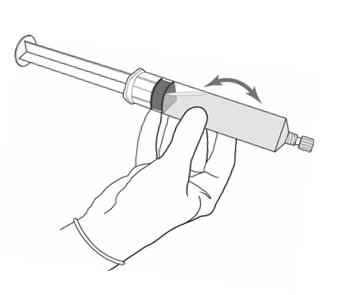
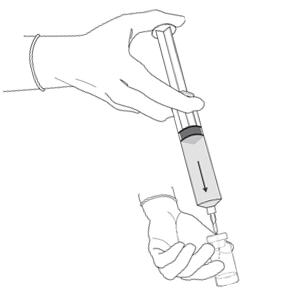
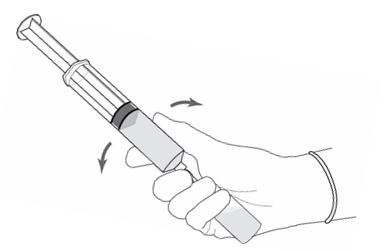
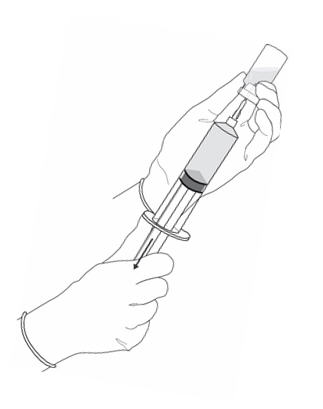
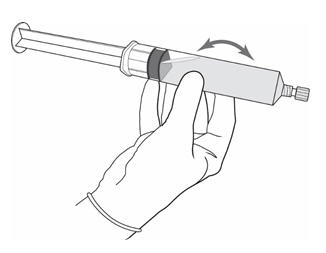
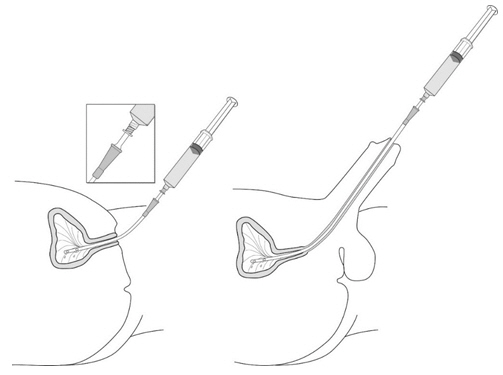
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - The recommended dose for adults is 50 mL of reconstituted solution of Cysview [ see - Dosage and Administration (2.2)], instilled into the ...
-
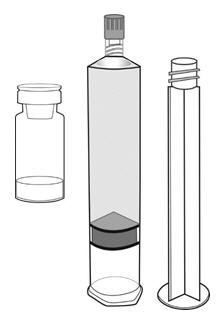
3 DOSAGE FORMS AND STRENGTHSCysview (hexaminolevulinate hydrochloride) is supplied as a kit. The kit may be supplied as two options; with or without a vial adapter, and contains: Cysview kit with a vial adapter ...
-
4 CONTRAINDICATIONSCysview is contraindicated in patients with: porphyria, gross hematuria, known hypersensitivity to hexaminolevulinate or any derivative of aminolevulinic acid.
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylaxis - Anaphylaxis, including anaphylactoid shock, has been reported following administration of Cysview [ see - Adverse Reactions (6.2)]. Prior to and ...
-
6 ADVERSE REACTIONSAnaphylaxis has been reported following exposure to Cysview [ see - Warnings and Precautions (5.1)]. 6.1 Clinical Study Experience - Because clinical trials ...
-
7 DRUG INTERACTIONSNo specific drug interaction studies have been performed.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Cysview use in pregnant women to inform a drug associated risk of adverse developmental outcomes. Adequate reproductive and ...
-
10 OVERDOSAGENo adverse events were reported in a dose-finding study conducted among patients whose bladders were instilled with twice the recommended concentration (dose) of solution of Cysview.
-
11 DESCRIPTIONCysview contains hexaminolevulinate hydrochloride, an optical imaging drug that in solution form is instilled intravesically for use with photodynamic blue light cystoscopy as an adjunct to white ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Cysview is an ester of the heme precursor, aminolevulinic acid. After bladder instillation, Cysview enters the bladder mucosa and is proposed to enter the intracellular ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies in animals have been conducted to evaluate the carcinogenic potential of hexaminolevulinate ...
-
14 CLINICAL STUDIESThe safety and efficacy of Cysview when used with photodynamic cystoscopy were studied in two controlled clinical trials. Study 1: A prospective, multicenter, controlled clinical trial in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCysview is supplied as a kit labeled Cysview (hexaminolevulinate HCl) Kit for Intravesical Solution, 100 mg. The kit may be supplied as two options; with or without a vial adapter, and contains ...
-
17 PATIENT COUNSELING INFORMATIONAsk patients if they have: a diagnosis or a family history of porphyria, allergy to aminolevulinic acid or prior exposure to Cysview, gross hematuria, had BCG immunotherapy or chemotherapy ...
-
SPL UNCLASSIFIED SECTIONDistributed by Photocure Inc. Princeton, NJ 08540 U.S.A. Cysview and BLC are registered trademarks of Photocure ASA with registration numbers 4021232 and 5461301, respectively. Photocure and the ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonCYSVIEW - ® (hexaminolevulinate HCl) KIT - for Intravesical Solution - 100 mg/vial - NDC 10511-3001-2 - ONE KIT - Sterile. Single ...
-
INGREDIENTS AND APPEARANCEProduct Information