Label: HETLIOZ- tasimelteon capsule
HETLIOZ LQ- tasimelteon suspension
- NDC Code(s): 43068-220-01, 43068-304-02, 43068-304-06
- Packager: Vanda Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HETLIOZ safely and effectively. See full prescribing information for HETLIOZ. HETLIOZ® (tasimelteon) capsules, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Non-24-Hour Sleep-Wake Disorder (Non-24) HETLIOZ capsules are indicated for the treatment of Non-24 in adults. 1.2 Nighttime Sleep Disturbances in Smith-Magenis Syndrome ...
-
2 DOSAGE AND ADMINISTRATION2.1 Non-Interchangeability between HETLIOZ Capsules and HETLIOZ LQ Oral Suspension - HETLIOZ capsules and HETLIOZ LQ oral suspension are not substitutable [see Clinical Pharmacology ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 20 mg size 1 dark blue opaque, hard gelatin capsules printed with “VANDA 20 mg” in white. Oral suspension: 4 mg/mL white to slightly yellow opaque suspension in 48 mL or 158 mL ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Somnolence - After taking HETLIOZ, patients should limit their activity to preparing for going to bed. HETLIOZ can potentially impair the performance of activities requiring complete mental ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Strong CYP1A2 Inhibitors (e.g., fluvoxamine) Avoid use of HETLIOZ in combination with fluvoxamine or other strong CYP1A2 inhibitors because of a potentially large increase in tasimelteon ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available postmarketing case reports with HETLIOZ use in pregnant women are not sufficient to evaluate drug-associated risk of major birth defects, miscarriage or ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Tasimelteon is not a controlled substance under the Controlled Substances Act. 9.2 Abuse - Tasimelteon did not produce any abuse-related signals in animal behavioral ...
-
10 OVERDOSAGEThere is limited premarketing clinical experience with the effects of an overdosage of HETLIOZ. As with the management of any overdose, general symptomatic and supportive measures should be used ...
-
11 DESCRIPTIONHETLIOZ (contains tasimelteon) a melatonin receptor agonist, chemically designated as (1R, 2R)-N-[2-(2,3-dihydrobenzofuran-4-yl)cyclopropylmethyl]propanamide, containing two chiral centers. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism by which tasimelteon exerts its therapeutic effect in patients with Non-24 or nighttime sleep disturbances in SMS is unclear. However, tasimelteon is an ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Tasimelteon was administered orally for up to two years to mice (30, 100, and 300 mg/kg/day) and rats (20, 100, and 250 ...
-
14 CLINICAL STUDIES14.1 Non-24-Hour Sleep-Wake Disorder (Non-24) The effectiveness of HETLIOZ in the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) was established in two randomized double-masked ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHETLIOZ Capsules - 20 mg capsules are available as size 1, dark blue opaque, hard gelatin capsules printed with “VANDA 20 mg” in white, containing 20 mg of tasimelteon per capsule. NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling for HETLIOZ LQ oral suspension, if appropriate (Instructions for Use). Advise patients to limit their activities to preparing for ...
-
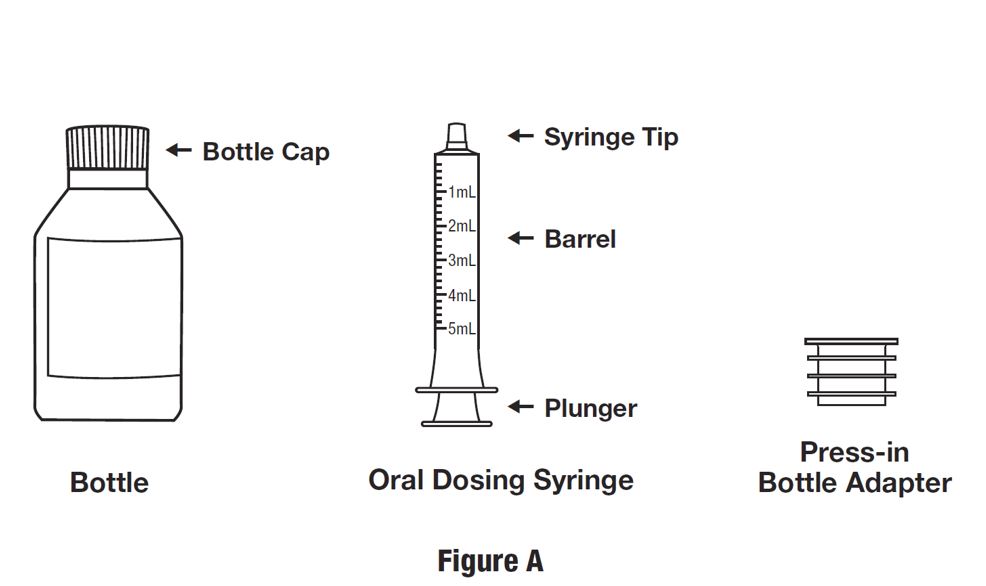
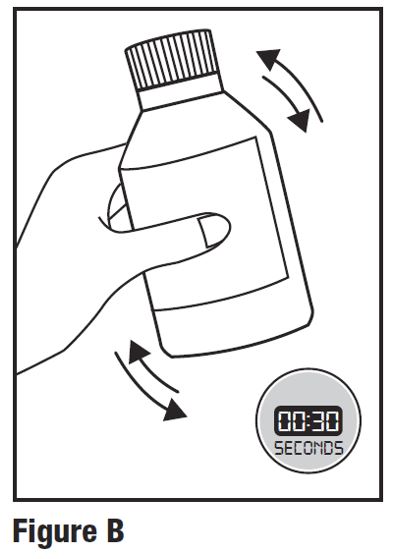
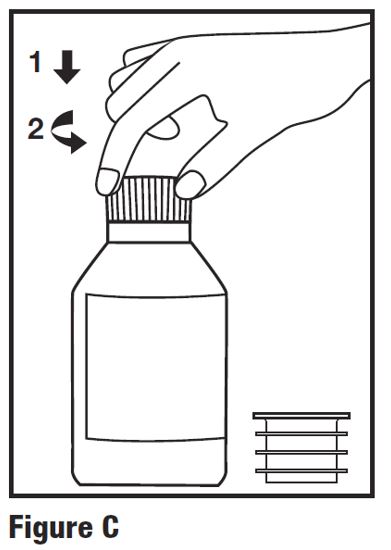
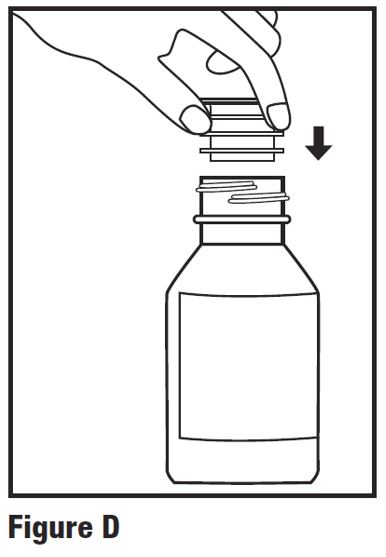
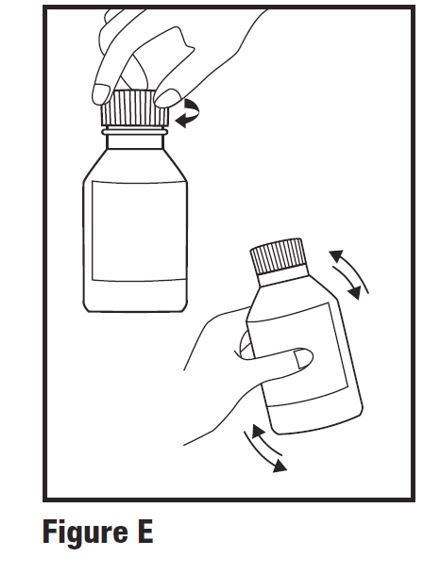
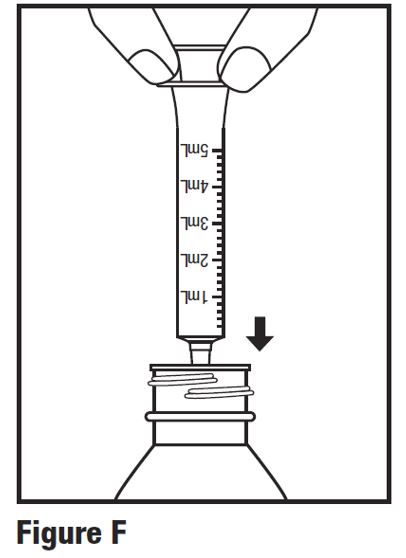
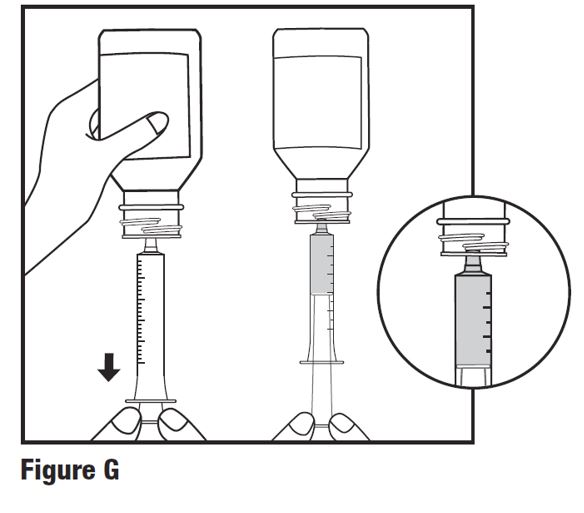
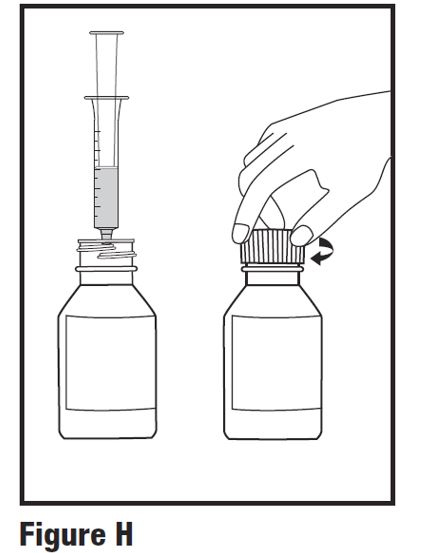
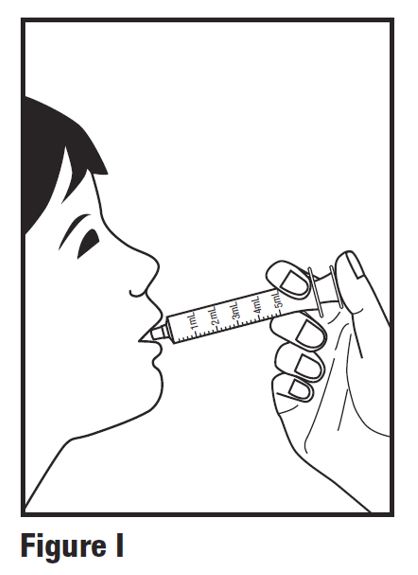
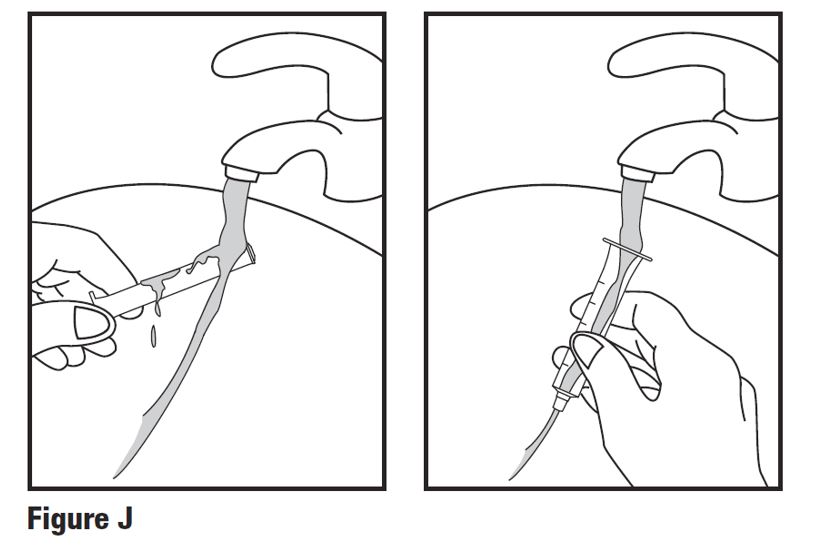
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - HETLIOZ LQ™ (HeT-lee-ōz eL-Cue) (tasimelteon) oral suspension - Read this Instructions for Use before you give HETLIOZ LQ to your child and each time you ...
-
PRINCIPAL DISPLAY PANEL - NDC 43068-220-01 - 20 mg Bottle

-
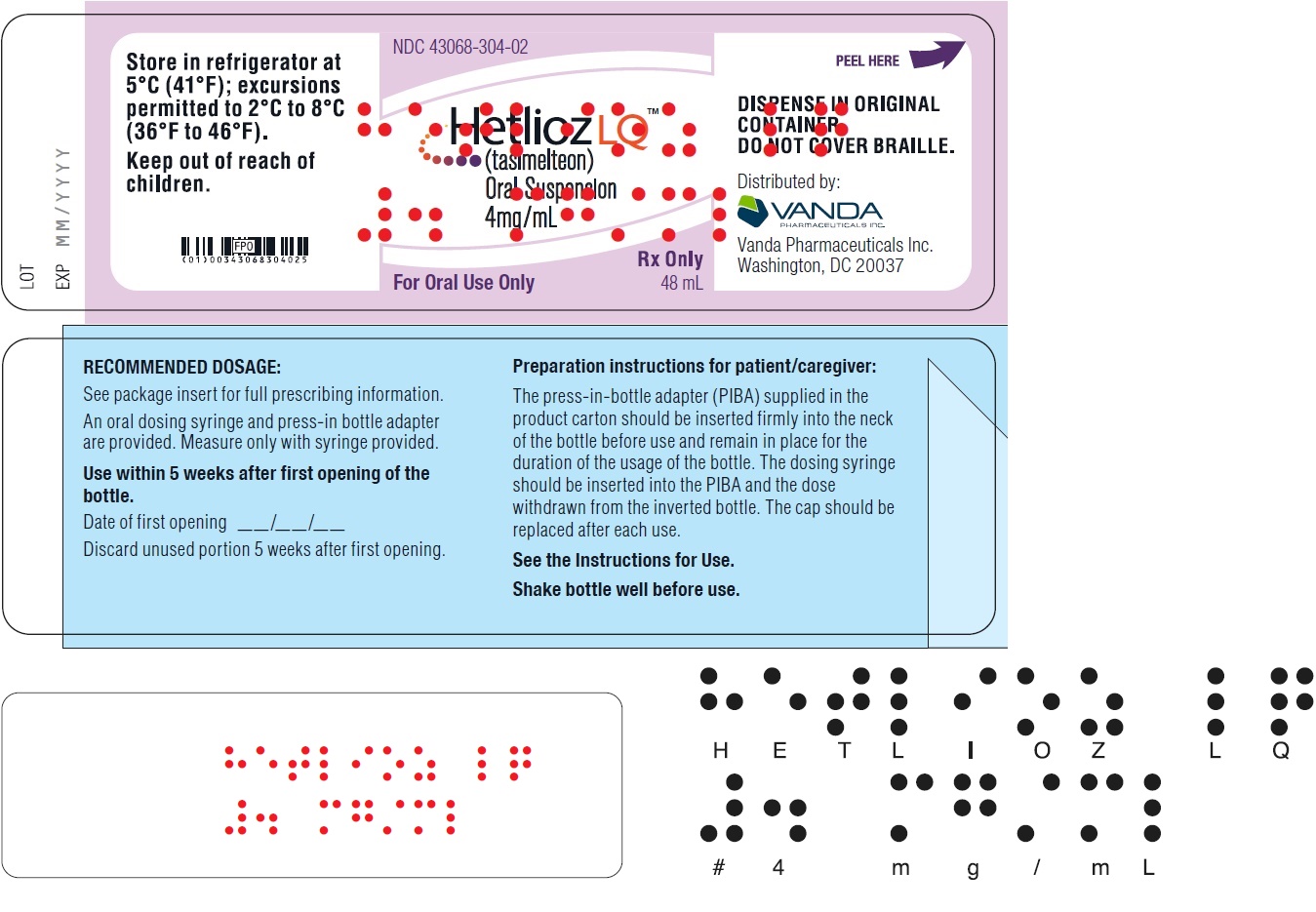
PRINCIPAL DISPLAY PANEL - NDC 43068-304-02 - 48 mL Bottle Label

-
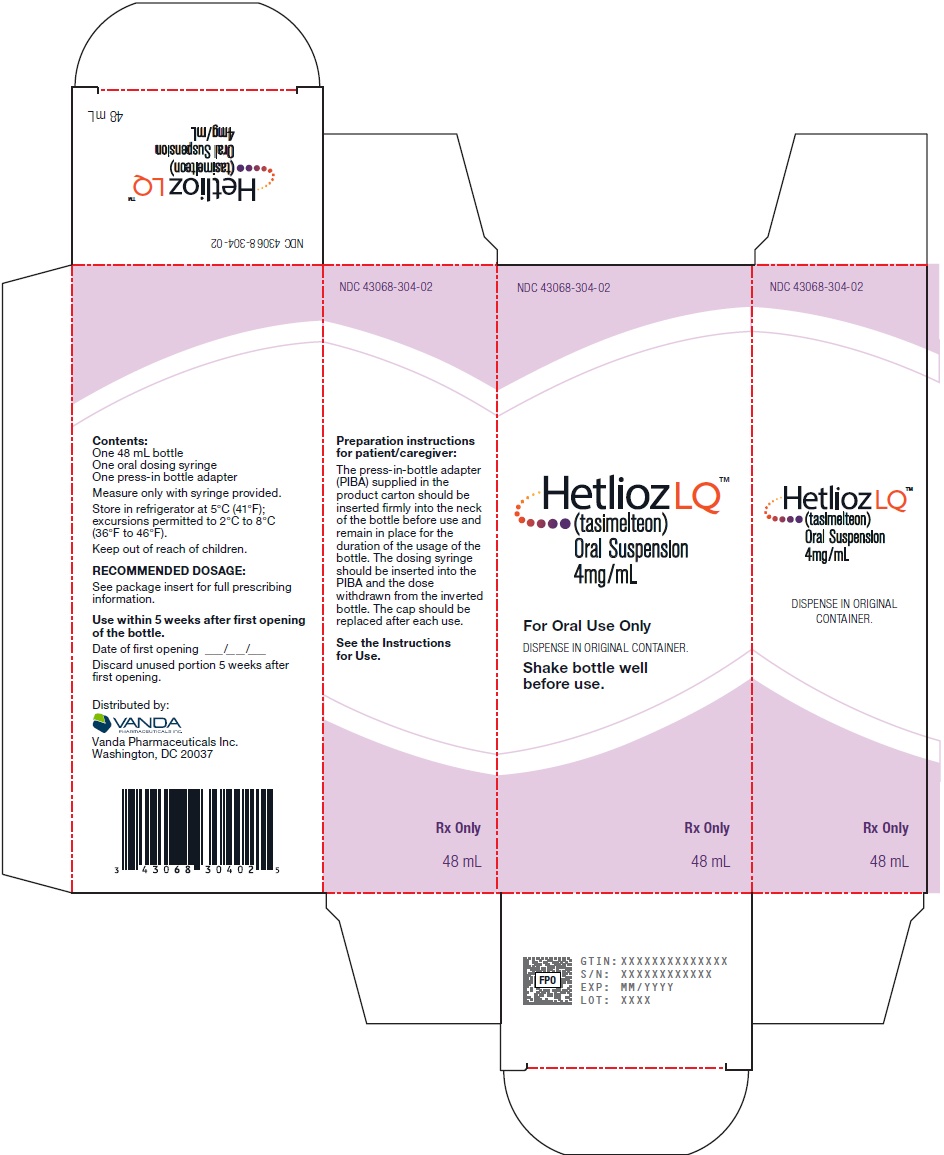
PRINCIPAL DISPLAY PANEL - NDC 43068-304-02 - 48 mL Carton Label

-
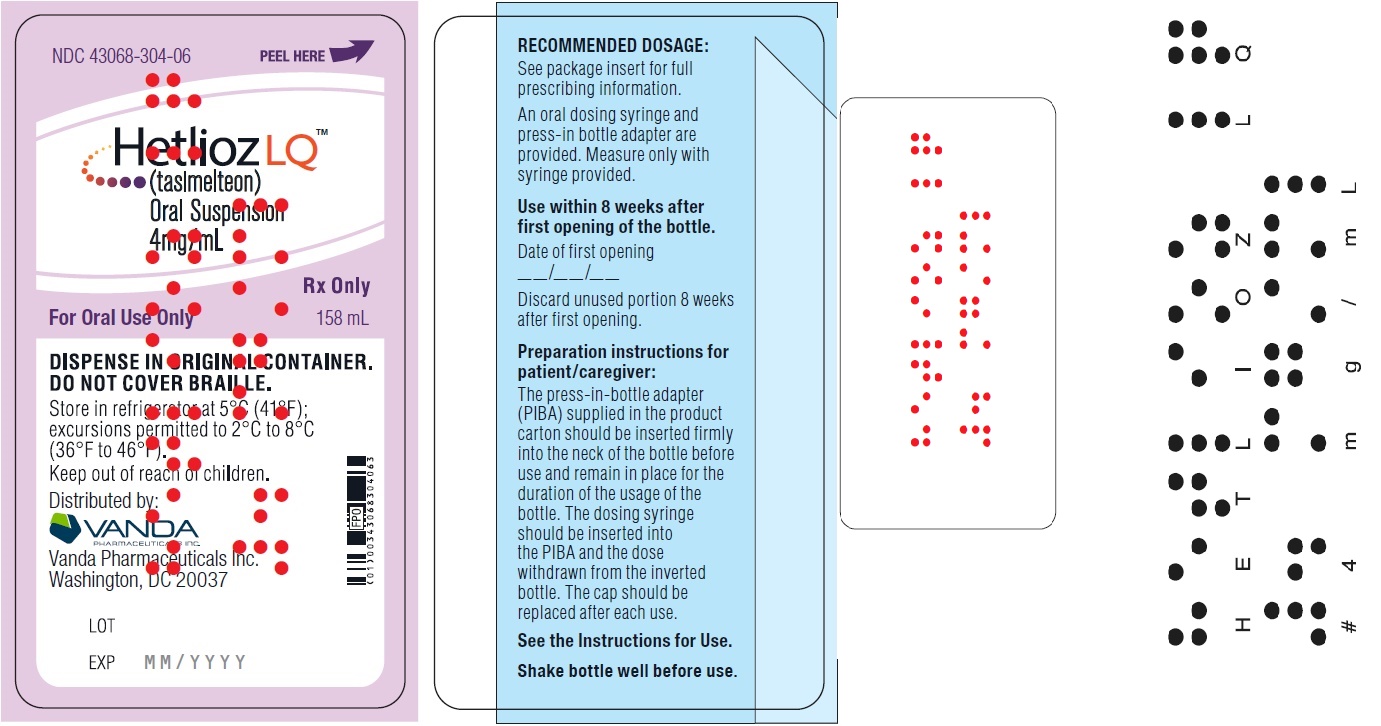
PRINCIPAL DISPLAY PANEL - NDC 43068-304-06 - 158 mL Bottle Label

-
PRINCIPAL DISPLAY PANEL - NDC 43068-304-06 - 158 mL Carton Label

-
INGREDIENTS AND APPEARANCEProduct Information