Label: HERCEPTIN HYLECTA- trastuzumab and hyaluronidase-oysk injection, solution
- NDC Code(s): 50242-077-01
- Packager: Genentech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HERCEPTIN HYLECTA safely and effectively. See full prescribing information for HERCEPTIN HYLECTA. HERCEPTIN HYLECTA® (trastuzumab ...These highlights do not include all the information needed to use HERCEPTIN HYLECTA safely and effectively. See full prescribing information for HERCEPTIN HYLECTA.
HERCEPTIN HYLECTA® (trastuzumab and hyaluronidase-oysk) injection, for subcutaneous use
Initial U.S. Approval: 2019WARNING: CARDIOMYOPATHY, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
See full prescribing information for complete boxed warning.
Cardiomyopathy: HERCEPTIN HYLECTA can result in subclinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue HERCEPTIN HYLECTA for cardiomyopathy. (2.4, 5.1)
Pulmonary Toxicity: Discontinue HERCEPTIN HYLECTA for anaphylaxis, angioedema, interstitial pneumonitis or acute respiratory distress syndrome. (5.3)
Embryo-Fetal Toxicity: Exposure to HERCEPTIN HYLECTA during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death. Advise patients of these risks and the need for effective contraception. (5.2, 8.1, 8.3)
RECENT MAJOR CHANGES
Dosage and Administration, Evaluation and Testing Before Initiating HERCEPTIN HYLECTA (2.1) 06/2024 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For subcutaneous use only. HERCEPTIN HYLECTA has different dosage and administration instructions than intravenous trastuzumab products.
Do not administer intravenously. (2.3)
Do not substitute HERCEPTIN HYLECTA for or with ado-trastuzumab emtansine. (2.3)
Perform HER2 testing using FDA-approved tests by laboratories with demonstrated proficiency. (1, 2.2)
The recommended dose of HERCEPTIN HYLECTA is 600 mg/10,000 units (600 mg trastuzumab and 10,000 units hyaluronidase) administered subcutaneously over approximately 2-5 minutes once every three weeks. (2.3)
DOSAGE FORMS AND STRENGTHS
- Injection: 600 mg trastuzumab and 10,000 units hyaluronidase per 5 mL (120 mg/2,000 units per mL) solution in a single-dose vial. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Exacerbation of Chemotherapy-Induced Neutropenia. (5.4, 6.1)
- Hypersensitivity and Administration-Related Reactions (ARRs): Severe ARRs, including anaphylaxis, have been reported with HERCEPTIN HYLECTA. Monitor patients for systemic hypersensitivity reactions. Permanently discontinue HERCEPTIN HYLECTA in patients who experience anaphylaxis or severe hypersensitivity reactions. (5.5)
ADVERSE REACTIONS
Adjuvant Breast Cancer
- Most common adverse reactions (≥10%) for HERCEPTIN HYLECTA are fatigue, arthralgia, diarrhea, injection site reaction, upper respiratory tract infection, rash, myalgia, nausea, headache, edema, flushing, pyrexia, cough, and pain in extremity. (6.1)
Metastatic Breast Cancer (based on intravenous trastuzumab)
- Most common adverse reactions (≥10%) are fever, chills, headache, infection, congestive heart failure, insomnia, cough, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Females and Males of Reproductive Potential: Verify the pregnancy status of females prior to initiation of HERCEPTIN HYLECTA. (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOMYOPATHY, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
1 INDICATIONS AND USAGE
1.1 Adjuvant Breast Cancer
1.2 Metastatic Breast Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Evaluation and Testing Before Initiating HERCEPTIN HYLECTA
2.2 Patient Selection
2.3 Recommended Dosage
2.4 Dosage Modification for Adverse Reactions
2.5 Administration and Storage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
5.2 Embryo-Fetal Toxicity
5.3 Pulmonary Toxicity
5.4 Exacerbation of Chemotherapy-Induced Neutropenia
5.5 Hypersensitivity and Administration-Related Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
14.2 Metastatic Breast Cancer
14.3 Patient Experience
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)Cardiomyopathy - HERCEPTIN HYLECTA administration can result in sub-clinical and clinical cardiac failure. The incidence and severity was highest in patients receiving HERCEPTIN HYLECTA with ...
WARNING: CARDIOMYOPATHY, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
Cardiomyopathy
HERCEPTIN HYLECTA administration can result in sub-clinical and clinical cardiac failure. The incidence and severity was highest in patients receiving HERCEPTIN HYLECTA with anthracycline-containing chemotherapy regimens.
Evaluate left ventricular function in all patients prior to and during treatment with HERCEPTIN HYLECTA. Discontinue HERCEPTIN HYLECTA treatment in patients receiving adjuvant therapy and withhold HERCEPTIN HYLECTA in patients with metastatic disease for clinically significant decrease in left ventricular function [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
Pulmonary Toxicity
HERCEPTIN HYLECTA administration can result in serious and fatal pulmonary toxicity. Symptoms usually occur during or within 24 hours of HERCEPTIN HYLECTA administration. Discontinue HERCEPTIN HYLECTA for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome [see Warnings and Precautions (5.3, 5.5)]. Monitor patients until symptoms completely resolve.
CloseEmbryo-Fetal Toxicity
Exposure to HERCEPTIN HYLECTA during pregnancy can result in oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death. Advise patients of these risks and the need for effective contraception [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE1.1 Adjuvant Breast Cancer - HERCEPTIN HYLECTA is indicated for adjuvant treatment of adults with HER2 overexpressing node positive or node negative (ER/PR negative or with one high risk feature ...
1.1 Adjuvant Breast Cancer
HERCEPTIN HYLECTA is indicated for adjuvant treatment of adults with HER2 overexpressing node positive or node negative (ER/PR negative or with one high risk feature [see Clinical Studies (14.1)]) breast cancer:
- as part of a treatment regimen consisting of doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel
- as part of a treatment regimen with docetaxel and carboplatin
- as a single agent following multi-modality anthracycline based therapy.
Select patients for therapy based on an FDA-approved companion diagnostic for trastuzumab [see Dosage and Administration (2.2)].
Close1.2 Metastatic Breast Cancer
HERCEPTIN HYLECTA is indicated in adults:
- In combination with paclitaxel for first-line treatment of HER2-overexpressing metastatic breast cancer
- As a single agent for treatment of HER2-overexpressing breast cancer in patients who have received one or more chemotherapy regimens for metastatic disease.
Select patients for therapy based on an FDA-approved companion diagnostic for trastuzumab [see Dosage and Administration (2.2)].
-
2 DOSAGE AND ADMINISTRATION2.1 Evaluation and Testing Before Initiating HERCEPTIN HYLECTA - Verify the pregnancy status of females of reproductive potential prior to the initiation of HERCEPTIN HYLECTA [see Use in ...
2.1 Evaluation and Testing Before Initiating HERCEPTIN HYLECTA
Verify the pregnancy status of females of reproductive potential prior to the initiation of HERCEPTIN HYLECTA [see Use in Specific Populations (8.1, 8.3)].
2.2 Patient Selection
Select patients based on HER2 protein overexpression or HER2 gene amplification in tumor specimens [see Indications and Usage (1) and Clinical Studies (14)]. Assessment of HER2 protein overexpression and HER2 gene amplification should be performed using FDA-approved tests specific for breast cancer by laboratories with demonstrated proficiency. Information on the FDA-approved tests for the detection of HER2 protein overexpression and HER2 gene amplification is available at: http://www.fda.gov/CompanionDiagnostics.
Improper assay performance, including use of suboptimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results.
2.3 Recommended Dosage
HERCEPTIN HYLECTA is for subcutaneous use only. HERCEPTIN HYLECTA has different dosage and administration instructions than intravenous trastuzumab products. Do not administer intravenously.
Do not substitute HERCEPTIN HYLECTA for or with ado-trastuzumab emtansine.
The recommended dose of HERCEPTIN HYLECTA is 600 mg/10,000 units (600 mg trastuzumab and 10,000 units hyaluronidase) administered subcutaneously over approximately 2-5 minutes once every three weeks.
No loading dose is required. No dose adjustments for patient body weight or for different concomitant chemotherapy regimens are required.
Duration of treatment
Patients with adjuvant breast cancer should be treated with HERCEPTIN HYLECTA for 52 weeks or until disease recurrence, whichever occurs first; extending treatment in adjuvant breast cancer beyond one year is not recommended.
Patients with metastatic breast cancer (MBC) should be treated with HERCEPTIN HYLECTA until progression of disease.
2.4 Dosage Modification for Adverse Reactions
Cardiomyopathy [see Boxed Warning, Warnings and Precautions (5.1)]
Assess left ventricular ejection fraction (LVEF) prior to initiation of HERCEPTIN HYLECTA and at regular intervals during treatment. Withhold HERCEPTIN HYLECTA dosing for at least 4 weeks for either of the following:
- ≥16% absolute decrease in LVEF from pre-treatment values
- LVEF below institutional limits of normal and ≥10% absolute decrease in LVEF from pretreatment values.
HERCEPTIN HYLECTA may be resumed if, within 4–8 weeks, the LVEF returns to normal limits and the absolute decrease from baseline is ≤15%.
Permanently discontinue HERCEPTIN HYLECTA for a persistent (>8 weeks) LVEF decline or for suspension of HERCEPTIN HYLECTA dosing on more than 3 occasions for cardiomyopathy.
Close2.5 Administration and Storage
To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is HERCEPTIN HYLECTA and not ado-trastuzumab emtansine or intravenous trastuzumab.
HERCEPTIN HYLECTA should be administered by a healthcare professional.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use vial if particulates or discoloration is present. Discard any unused portion remaining in the vial.
HERCEPTIN HYLECTA is for single use only. The 600 mg/10,000 units (600 mg trastuzumab and 10,000 units hyaluronidase) solution is a ready to use solution for injection which does not need to be diluted.
To avoid needle clogging, attach the hypodermic injection needle to the syringe immediately prior to administration followed by volume adjustment to 5 mL. HERCEPTIN HYLECTA is compatible with polypropylene and polycarbonate syringe material and stainless steel transfer and injection needles.
Prepare the dosing syringe in controlled and validated aseptic conditions. After the solution of HERCEPTIN HYLECTA is withdrawn from the vial and into the syringe, replace the transfer needle with a syringe closing cap. Label the syringe with the peel-off sticker.
Administration
The injection site should be alternated between the left and right thigh. New injections should be given at least 2.5 cm from the old previous site on healthy skin and never into areas where the skin is red, bruised, tender, or hard, or areas where there are moles or scars. During the treatment course with HERCEPTIN HYLECTA other medicinal products for subcutaneous administration should preferably be injected at different sites. The dose should be administered subcutaneously over approximately 2 to 5 minutes.
-
3 DOSAGE FORMS AND STRENGTHSHERCEPTIN HYLECTA is a colorless to yellowish, clear to opalescent solution for subcutaneous injection: Injection: 600 mg trastuzumab and 10,000 units hyaluronidase per 5 mL (120 mg/2,000 units ...
HERCEPTIN HYLECTA is a colorless to yellowish, clear to opalescent solution for subcutaneous injection:
- Injection: 600 mg trastuzumab and 10,000 units hyaluronidase per 5 mL (120 mg/2,000 units per mL) in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiomyopathy - HERCEPTIN HYLECTA can cause left ventricular cardiac dysfunction, arrhythmias, hypertension, disabling cardiac failure, cardiomyopathy, and cardiac death [see Boxed Warning ...
5.1 Cardiomyopathy
HERCEPTIN HYLECTA can cause left ventricular cardiac dysfunction, arrhythmias, hypertension, disabling cardiac failure, cardiomyopathy, and cardiac death [see Boxed Warning: Cardiomyopathy]. HERCEPTIN HYLECTA can also cause asymptomatic decline in LVEF.
There is a 4–6 fold increase in the incidence of symptomatic myocardial dysfunction among patients receiving trastuzumab as a single agent or in combination therapy compared with those not receiving trastuzumab. The highest absolute incidence occurs when trastuzumab is administered with an anthracycline. The incidence of symptomatic myocardial dysfunction for intravenous trastuzumab and HERCEPTIN HYLECTA was similar in clinical trials [see Adverse Reactions (6)].
Withhold HERCEPTIN HYLECTA for ≥16% absolute decrease in LVEF from pre-treatment values or an LVEF value below institutional limits of normal and ≥10% absolute decrease in LVEF from pretreatment values [see Dosage and Administration (2.4)]. The safety of continuation or resumption of HERCEPTIN HYLECTA in patients with HERCEPTIN HYLECTA induced left ventricular cardiac dysfunction has not been studied.
Patients who receive anthracycline after stopping HERCEPTIN HYLECTA may also be at increased risk of cardiac dysfunction [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
Cardiac Monitoring
Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan. The following schedule is recommended:
- Baseline LVEF measurement immediately prior to initiation of HERCEPTIN HYLECTA
- LVEF measurements every 3 months during and upon completion of HERCEPTIN HYLECTA
- Repeat LVEF measurement at 4 week intervals if HERCEPTIN HYLECTA is withheld for significant left ventricular cardiac dysfunction [see Dosage and Administration (2.4)]
- LVEF measurements every 6 months for at least 2 years following completion of HERCEPTIN HYLECTA as a component of adjuvant therapy.
HERCEPTIN HYLECTA
In the HannaH study, the overall percentage of patients with at least one cardiac disorder was similar in both study arms: 15% (44/297) of patients in the HERCEPTIN HYLECTA arm and 14% (42/298) of patients in the intravenous trastuzumab arm. The most frequent cardiac adverse reactions were left ventricular dysfunction [3.4% (10/297) and 4.0% (12/298)], tachycardia [2% (6/297) and 3% (9/298)] and palpitations [2% (6/297) and 1.3% (4/298)] in the HERCEPTIN HYLECTA arm and the intravenous trastuzumab arm, respectively. The incidence of cardiac failure and congestive cardiac failure was 1% (3/297) in the HERCEPTIN HYLECTA arm and <1% (1/298) in the intravenous trastuzumab arm. The proportion of patients in each treatment arm with a significant decrease in LVEF defined as a drop of ≥10% points to an LVEF of <50% was comparable between treatment arms [3.8% (11/297) in the HERCEPTIN HYLECTA arm and 4.2% (12/298) in the intravenous trastuzumab arm]. In patients with lower body weights (<59 kg, the lowest body weight quartile) the fixed-dose used in the HERCEPTIN HYLECTA arm was not associated with an increased risk of cardiac events or significant drop in LVEF.
In the SafeHER study, in patients treated with HERCEPTIN HYLECTA, 17% (323/1864) reported a cardiac disorder during the treatment period. Decreased ejection fraction, reported in 4.5% (84/1864) of the patients was the most frequently reported cardiac disorder. Congestive cardiac failure was reported in <1% (10/1864) of patients and <1% (4/1864) of patients reported cardiac failure during the treatment period. One patient reported congestive cardiac failure during the follow-up period. Six percent (111/1864) of the patients treated with HERCEPTIN HYLECTA had an LVEF <50% with a decrease of ≥10 points in LVEF from baseline.
Trastuzumab (intravenous formulation):
In study NSABP B31 (NCT00004067), 15% (158/1031) of patients discontinued intravenous trastuzumab due to clinical evidence of myocardial dysfunction or significant decline in LVEF after a median follow-up duration of 8.7 years in the AC-TH arm. In the HERA study (one-year intravenous trastuzumab treatment; NCT00045032), the number of patients who discontinued intravenous trastuzumab due to cardiac toxicity at 12.6 months median duration of follow-up was 2.6% (44/1678). In the BCIRG006 study (NCT00021255), a total of 2.9% (31/1056) of patients in the TCH arm (1.5% during the chemotherapy phase and 1.4% during the monotherapy phase) and 5.7% (61/1068) of patients in the AC-TH arm (1.5% during the chemotherapy phase and 4.2% during the monotherapy phase) discontinued intravenous trastuzumab due to cardiac toxicity.
Among 64 patients receiving adjuvant chemotherapy (studies NSABP B31 and NCCTG N9831; NCT00005970) who developed congestive heart failure (CHF), one patient died of cardiomyopathy, one patient died suddenly without documented etiology, and 33 patients were receiving cardiac medication at last follow-up. Approximately 24% of the surviving patients had recovery to a normal LVEF (defined as ≥ 50%) and no symptoms on continuing medical management at the time of last follow-up. Incidence of CHF is presented in Table 1. The safety of continuation or resumption of intravenous trastuzumab in patients with trastuzumab-induced left ventricular cardiac dysfunction has not been studied.
Table 1 Incidence of Congestive Heart Failure in Adjuvant Breast Cancer Studies Incidence of Congestive Heart Failure
% (n)Study Regimen Intravenous Trastuzumab Control - *
- Median follow-up duration for studies NSABP B31 and NCCTG N9831 combined was 8.3 years in the AC→ paclitaxel+Herceptin arm.
- †
- Anthracycline (doxorubicin) and cyclophosphamide.
- ‡
- Includes 1 patient with fatal cardiomyopathy and 1 patient with sudden death without documented etiology.
- §
- Includes NYHA II-IV and cardiac death at 12.6 months median duration of follow-up in the one-year intravenous trastuzumab arm.
NSABP B31 & NCCTG N9831* AC†→paclitaxel + intravenous trastuzumab 3.2% (64/2000) ‡ 1.3% (21/1655) HERA§ Chemotherapy → intravenous trastuzumab 2% (30/1678) 0.3% (5/1708) BCIRG006 AC†→docetaxel + intravenous trastuzumab 2% (20/1068) 0.3% (3/1050) BCIRG006 Docetaxel + carboplatin + intravenous trastuzumab 0.4% (4/1056) 0.3% (3/1050) In the HERA study (one-year intravenous trastuzumab treatment), at a median follow-up duration of 8 years, the incidence of severe CHF (NYHA III & IV) was 0.8%, and the rate of mild symptomatic and asymptomatic left ventricular dysfunction was 4.6%.
Table 2 Incidence of Cardiac Dysfunction* in Metastatic Breast Cancer Studies Incidence NYHA I–IV NYHA III–IV Study Event Intravenous Trastuzumab Control Intravenous Trastuzumab Control H0648g
(AC)†Cardiac Dysfunction 28% 7% 19% 3% H0648g
(paclitaxel)Cardiac Dysfunction 11% 1% 4% 1% H0649g Cardiac Dysfunction‡ 7% N/A 5% N/A In the BCIRG006 study, the incidence of NCI-CTC Grade 3/4 cardiac ischemia/infarction was higher in the intravenous trastuzumab containing regimens [AC-TH: 0.3% (3/1068) and TCH: 0.2% (2/1056)] as compared to none in AC-T.
5.2 Embryo-Fetal Toxicity
HERCEPTIN HYLECTA can cause fetal harm when administered to a pregnant woman. In post-marketing reports, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
Verify the pregnancy status of females of reproductive potential prior to the initiation of HERCEPTIN HYLECTA. Advise pregnant women and females of reproductive potential that exposure to HERCEPTIN HYLECTA during pregnancy or within 7 months prior to conception can result in fetal harm. Advise females of reproductive potential to use effective contraception during treatment and for 7 months following the last dose of HERCEPTIN HYLECTA [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.3)].
5.3 Pulmonary Toxicity
HERCEPTIN HYLECTA can result in serious and fatal pulmonary toxicity. Pulmonary toxicity includes dyspnea, interstitial pneumonitis, pulmonary infiltrates, pleural effusions, non-cardiogenic pulmonary edema, pulmonary insufficiency and hypoxia, acute respiratory distress syndrome, and pulmonary fibrosis. Patients with symptomatic intrinsic lung disease or with extensive tumor involvement of the lungs, resulting in dyspnea at rest, appear to have more severe toxicity.
5.4 Exacerbation of Chemotherapy-Induced Neutropenia
HERCEPTIN HYLECTA may exacerbate chemotherapy-induced neutropenia. In randomized, controlled clinical trials with intravenous trastuzumab, the per-patient incidences of NCI-CTC Grade 3–4 neutropenia and of febrile neutropenia were higher in patients receiving trastuzumab in combination with myelosuppressive chemotherapy as compared to those who received chemotherapy alone. The incidence of septic death was similar among patients who received trastuzumab and those who did not [see Adverse Reactions (6.1)].
Close5.5 Hypersensitivity and Administration-Related Reactions
Severe administration-related reactions (ARRs), including hypersensitivity and anaphylaxis, have been reported with HERCEPTIN HYLECTA. Patients experiencing dyspnea at rest due to complications of advanced malignancy and comorbidities may be at increased risk of a severe or of a fatal ARR.
In the HannaH and SafeHER trials, 9% and 4.2% of patients experienced Grade 1-4 hypersensitivity and anaphylaxis, respectively. Grade 3-4 hypersensitivity and anaphylactic reactions occurred in 1% and <1% of the patients treated with HERCEPTIN HYLECTA, respectively. In the SafeHER trial, 2 patients required permanent treatment discontinuation with HERCEPTIN HYLECTA (1 patient due to a hypersensitivity reaction and 1 patient due to anaphylaxis). Serious and fatal reactions have been reported after treatment with intravenous trastuzumab products.
Closely monitor patients for systemic hypersensitivity reactions, especially during the first administration. Permanently discontinue HERCEPTIN HYLECTA in patients who experience anaphylaxis or severe hypersensitivity reactions. Medications to treat such reactions, as well as emergency equipment, should be available for immediate use. For patients experiencing reversible Grade 1 or 2 hypersensitivity reactions, consider pre-medication with an analgesic, antipyretic, or an antihistamine prior to readministration of HERCEPTIN HYLECTA [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: Cardiomyopathy [see Warnings and Precautions (5.1)] Embryo-Fetal Toxicity [see Warnings and ...
The following adverse reactions are discussed in greater detail in other sections of the label:
- Cardiomyopathy [see Warnings and Precautions (5.1)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.2)]
- Pulmonary Toxicity [see Warnings and Precautions (5.3)]
- Exacerbation of Chemotherapy-Induced Neutropenia [see Warnings and Precautions (5.4)]
- Hypersensitivity and Administration-Related Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of HERCEPTIN HYLECTA administered subcutaneously has been established in the HannaH and SafeHER studies conducted in patients with HER2 overexpressing breast cancer. The safety of intravenous trastuzumab has been established in studies H0648g and H0649g conducted in patients with HER2 overexpressing metastatic breast cancer.
Adjuvant Breast Cancer
HannaH
HannaH was a randomized, open-label study to compare the pharmacokinetics, efficacy, and safety of HERCEPTIN HYLECTA compared to intravenous trastuzumab in women with HER2-positive breast cancer. Patients randomized to the HERCEPTIN HYLECTA arm received a dose of 600 mg HERCEPTIN HYLECTA every 3 weeks throughout the treatment phase. Patients were treated for 8 cycles in combination with chemotherapy (docetaxel followed by 5FU, epirubicin and cyclophosphamide), then underwent surgery, and continued HERCEPTIN HYLECTA to complete 18 cycles of therapy. The median age of patients was 50 (range: 25-81 years), all patients were female, and a majority of patients were white (67%). The median number of HERCEPTIN HYLECTA cycles received was 18 (range 1-18).
The most common adverse reactions of any grade (occurring in ≥10% of patients) with HERCEPTIN HYLECTA were alopecia (63%), nausea (49%), ARRs (48%), neutropenia (44%), diarrhea (34%), asthenia (25%), fatigue (24%), vomiting (23%), myalgia (21%), decreased appetite (20%), stomatitis (19%), arthralgia (18%), headache (17%), rash (16%), constipation (14%), radiation skin injury (14%), pyrexia (12%), cough (12%), anemia (11%), dyspnea (11%), incision site pain (11%), peripheral sensory neuropathy (11%), leukopenia (10%), mucosal inflammation (10%), hot flush (10%), upper respiratory tract infection (10%).
The most common Grade ≥3 adverse reactions (occurring in >1% of patients) in the HERCEPTIN HYLECTA arm were neutropenia (30%), febrile neutropenia (6%), leukopenia (4%), diarrhea (3%), hypertension (2%), irregular menstruation (2%), alopecia (1%), nausea (1%), granulocytopenia (1%), vomiting (1%), amenorrhea (1%), and cellulitis (1%). Adverse reactions leading to interruption of any study drug in the HERCEPTIN HYLECTA arm occurred in 34% of patients; 31% of patients had these events during the neoadjuvant phase of the study with concurrent chemotherapy and 9% of patients had these events during the adjuvant phase. Overall, the most common (≥ 1%) were neutropenia (21%), leukopenia (2.4%), ALT increase (1.7%), pyrexia (1.7%), anemia (1%), bronchitis (1%), and left ventricular dysfunction (1%). Adverse reactions that led to discontinuation of any study drug in the HERCEPTIN HYLECTA arm (>1 patient) were left ventricular dysfunction (2%).
The incidence of ARRs in the HERCEPTIN HYLECTA arm was 48% and was 37% in the intravenous trastuzumab arm. Five (2%) patients in the HERCEPTIN HYLECTA arm experienced a Grade 3 ARR. Three of the events in the HERCEPTIN HYLECTA arm occurred on the day of study drug administration when docetaxel treatment was administered concurrently. The most commonly reported ARRs in the HERCEPTIN HYLECTA arm (≥5% of patients) were rash, pruritus, erythema, cough and dyspnea. Grade 1 and 2 injection-site reactions (ISRs) occurred in 10% of patients in the HERCEPTIN HYLECTA arm. The most common ISRs were injection-site pain and injection-site erythema.
The data in Table 3 were obtained from the HannaH trial for adverse reactions that occurred in ≥ 5% of the patients treated with HERCEPTIN HYLECTA.
Table 3 Adverse Reactions* (≥ 5% Incidence) Reported in HannaH Adverse Reactions HERCEPTIN HYLECTA
600 mg
n=297Intravenous Trastuzumab
(loading dose: 8 mg/kg; maintenance dose: 6 mg/kg)
n=298All Grades
%Grades 3 to 5
%All Grades
%Grades 3 to 5
%- *
- Contains grouped terms
- †
- The HannaH trial was not designed to demonstrate a statistically significant difference in adverse reaction rates between HERCEPTIN HYLECTA and intravenous trastuzumab.
- ‡
- Injection Site Reaction includes terms for injection related reaction and injection site joint pain, bruising, dermatitis, discoloration, discomfort, erythema, extravasation, fibrosis, hematoma, hemorrhage, hypersensitivity, induration, inflammation, irritation, macule, mass, nodule, edema, pallor, paraesthesia, pruritus, rash, reaction, swelling, ulcer, vesicles and warmth.
SKIN AND SUBCUTANEOUS TISSUE DISORDERS Alopecia*,† 63 1.3 63 1.7 Rash*,† 26 < 1 26 - Nail Disorder*,† 14 - 14 < 1 Pruritus*,† 9 - 9 - Skin Discoloration* 9 - 8 - Erythema* 7 < 1 3 - GASTROINTESTINAL DISORDERS Nausea 49 1.3 49 1.3 Diarrhea*,† 34 2.7 37 2.7 Vomiting† 23 1 23 1.7 Stomatitis* 21 < 1 18 < 1 Abdominal Pain*,† 14 - 14 < 1 Dyspepsia 11 - 10 - GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS Fatigue*,† 46 < 1 49 2 Edema*,† 14 - 15 - Pyrexia* 13 1 12 < 1 Mucosal Inflammation† 10 < 1 13 - Pain*,† 5 - 8 < 1 Injection Site Reaction*,‡ 10 - < 1 - BLOOD AND LYMPHATIC SYSTEM DISORDERS Neutropenia† 44 30 47 34 Leukopenia*,† 11 5 16 8 Anemia*,† 12 < 1 14 1 Febrile Neutropenia* 6 6 4 4 INFECTIONS AND INFESTATIONS Upper Respiratory Tract Infection*,† 24 1 27 < 1 Urinary Tract Infection*,† 4 - 8 < 1 MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS Myalgia* 21 - 19 < 1 Arthralgia*,† 18 - 21 < 1 Back Pain* 11 1 9 1 Pain in Extremity 10 - 9 < 1 Pain*,† 8 < 1 9 - Bone Pain 6 < 1 3.4 - NERVOUS SYSTEM DISORDERS Neuropathy Peripheral* 20 - 15 - Headache* 17 < 1 15 < 1 Dizziness* 10 < 1 9 < 1 Dysgeusia* 10 - 8 - INJURY, POISONING AND PROCEDURAL COMPLICATIONS Incision Site Complication* 11 - 8 < 1 Pain* 6 - 5 < 1 RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS Cough* 12 < 1 8 - Dyspnea*,† 7 - 8 - Epistaxis 6 - 6 - Nasal Inflammation / Discomfort*,† 5 - 7 - VASCULAR DISORDERS Flushing* 14 < 1 13 < 1 Hypertension* 8 2.4 5 < 1 METABOLISM AND NUTRITION DISORDERS Decreased Appetite 20 < 1 20 < 1 INVESTIGATIONS Liver Function Analysis Abnormal*,† 6 1 9 1.7 CARDIAC DISORDERS Arrhythmia*† 5 - 5 < 1 IMMUNE SYSTEM DISORDERS Hypersensitivity*,† 7 1 7 1.3 SafeHER
SafeHER was a prospective, two-cohort, non-randomized, multi-center, multinational, open-label study to assess the safety of HERCEPTIN HYLECTA in patients with operable HER2-positive breast cancer. In SafeHER, 1864 patients were enrolled and treated with 600 mg of HERCEPTIN HYLECTA administered subcutaneously once every three weeks for 18 cycles.
The median age of patients was 54 (range: 20-88 years), 99.8% were female, and a majority were white (76%). A majority of the patients received HERCEPTIN HYLECTA concurrently with a chemotherapy regimen (58%). The median number of HERCEPTIN HYLECTA cycles administered was 18 and the median duration of HERCEPTIN HYLECTA exposure was 11.8 months. The median duration of follow-up was 23.7 months.
During the treatment period, the most common adverse reactions of any grade (occurring in ≥10% of patients) were ARRs (39%), diarrhea (21%), fatigue (21%), arthralgia (21%), nausea (15%), myalgia (14%), headache (13%), asthenia (12%), pain in extremity (11%), cough (11%), pyrexia (11%), hot flush (10%), and rash (10%). The most common Grade ≥3 adverse reactions (occurring in >1% of patients) were neutropenia (4%), febrile neutropenia (2%), hypertension (2%), leukopenia (1%), and diarrhea (1%). Adverse reactions that led to study drug discontinuation (≥0.5% of patients) were ejection fraction decreased (2%) and left ventricular dysfunction (1%).
The incidence of ARRs was 39%, with Grade ≥3 ARRs reported in 1% of patients treated with HERCEPTIN HYLECTA. The most frequently reported Grade ≥3 ARRs were dyspnea (<1%), cough (<1%), erythema (<1%), rash (<1%), and drug hypersensitivity (<1%). ISRs were reported in 20% of patients treated with HERCEPTIN HYLECTA. The most common ISRs were injection-site erythema (7%) and injection-site pain (6%). All ISRs were Grade 1 or 2, except for one (<1%) Grade 3 injection site discomfort.
The data in Table 4 were obtained from the SafeHER trial for adverse reactions that occurred in ≥5% of the patients treated with HERCEPTIN HYLECTA.
Table 4 Adverse Reactions* (≥ 5% Incidence) Reported in SafeHER Adverse Reactions *,† HERCEPTIN HYLECTA
600 mg (once every 3 weeks)
n=1864All Grades
%Grades 3 to 5
%- *
- Contains grouped terms
- †
- Includes adverse reactions reported throughout study treatment and follow-up.
- ‡
- ISR includes injection related reaction and injection site joint pain, bruising, dermatitis, discoloration, discomfort, erythema, extravasation, fibrosis, hematoma, hemorrhage, hypersensitivity, induration, inflammation, irritation, macule, mass, nodule, edema, pallor, paresthesia, pruritus, rash, reaction, swelling, ulcer, vesicles and warmth.
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS Fatigue* 33 < 1 Injection Site Reaction*,‡ 20 < 1 Edema* 12 < 1 Pyrexia* 11 < 1 Pain* 8 < 1 Mucosal Inflammation 6 < 1 MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS Arthralgia* 21 < 1 Myalgia* 17 < 1 Pain in Extremity 11 < 1 Back Pain* 8 < 1 Pain* 7 < 1 GASTROINTESTINAL DISORDERS Diarrhea* 21 1 Nausea 15 < 1 Abdominal Pain* 10 < 1 Constipation 9 < 1 Stomatitis* 8 < 1 Vomiting 7 < 1 SKIN AND SUBCUTANEOUS TISSUE DISORDERS Rash* 17 < 1 Nail Disorder* 10 < 1 Alopecia* 9 < 1 Erythema* 9 < 1 Pruritus* 6 - INFECTIONS AND INFESTATIONS Upper Respiratory Tract Infection* 19 < 1 Urinary Tract Infection* 6 < 1 Viral Infection* 5 - NERVOUS SYSTEM DISORDERS Neuropathy Peripheral* 14 < 1 Headache* 13 < 1 Dizziness* 6 < 1 Paresthesia 6 < 1 RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS Cough* 11 < 1 Dyspnea* 8 < 1 Epistaxis 6 - Nasal Inflammation/Discomfort* 6 - VASCULAR DISORDERS Flushing* 12 < 1 Hypertension* 8 2 BLOOD AND LYMPHATIC SYSTEM DISORDERS Anemia* 8 < 1 Neutropenia 6 4 PSYCHIATRIC DISORDERS Insomnia* 7 < 1 Metastatic Breast Cancer (based on intravenous trastuzumab)
The data below reflect exposure to intravenous trastuzumab in one randomized, open-label study, H0648g, of chemotherapy with (n=235) or without (n=234) intravenous trastuzumab in patients with metastatic breast cancer, and one single-arm study (H0649g; n=222) in patients with metastatic breast cancer. Data in Table 5 are based on H0648g and H0649g.
Among the 464 patients treated in H0648g, the median age was 52 years (range: 25–77 years). Eighty-nine percent were white, 5% black, 1% Asian, and 5% other racial/ethnic groups. All patients received 4 mg/kg initial dose of intravenous trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received intravenous trastuzumab treatment for ≥ 6 months and ≥ 12 months were 58% and 9%, respectively.
Among the 352 patients treated in single agent studies (213 patients from H0649g), the median age was 50 years (range 28–86 years), 86% were white, 3% were black, 3% were Asian, and 8% in other racial/ethnic groups. Most of the patients received 4 mg/kg initial dose of intravenous trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received intravenous trastuzumab treatment for ≥ 6 months and ≥ 12 months were 31% and 16%, respectively.
Table 5 Adverse Reactions ( ≥ 5%) in the Intravenous Trastuzumab Arm (H0648g and H0649g) Intravenous trastuzumab *
n = 352
%Intravenous trastuzumab + Paclitaxel
n = 91
%Paclitaxel
n = 95
%Intravenous trastuzumab + AC†
n = 143
%AC†
n = 135
%General Pain 47 61 62 57 42 Asthenia 42 62 57 54 55 Fever 36 49 23 56 34 Chills 32 41 4 35 11 Headache 26 36 28 44 31 Abdominal pain 22 34 22 23 18 Back pain 22 34 30 27 15 Infection 20 47 27 47 31 Flu syndrome 10 12 5 12 6 Accidental injury 6 13 3 9 4 Allergic reaction 3 8 2 4 2 Gastrointestinal Nausea 33 51 9 76 77 Diarrhea 25 45 29 45 26 Vomiting 23 37 28 53 49 Anorexia 14 24 16 31 26 Nausea and vomiting 8 14 11 18 9 Respiratory Cough increased 26 41 22 43 29 Dyspnea 22 27 26 42 25 Rhinitis 14 22 5 22 16 Pharyngitis 12 22 14 30 18 Sinusitis 9 21 7 13 6 Skin Rash 18 38 18 27 17 Herpes simplex 2 12 3 7 9 Acne 2 11 3 3 < 1 Nervous Insomnia 14 25 13 29 15 Dizziness 13 22 24 24 18 Paresthesia 9 48 39 17 11 Depression 6 12 13 20 12 Peripheral neuritis 2 23 16 2 2 Neuropathy 1 13 5 4 4 Metabolic Peripheral edema 10 22 20 20 17 Edema 8 10 8 11 5 Cardiovascular Congestive heart failure 7 11 1 28 7 Tachycardia 5 12 4 10 5 Musculoskeletal Bone pain 7 24 18 7 7 Arthralgia 6 37 21 8 9 Urogenital Urinary tract infection 5 18 14 13 7 Blood and Lymphatic Anemia 4 14 9 36 26 Leukopenia 3 24 17 52 34 Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of trastuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Administration-related reaction [see Warnings and Precautions (5.5)]
- Oligohydramnios or oligohydramnios sequence, including pulmonary hypoplasia, skeletal abnormalities, and neonatal death [see Warnings and Precautions (5.2)]
- Glomerulopathy [see Adverse Reactions (6.1)]
- Immune thrombocytopenia
- Tumor lysis syndrome (TLS): Cases of possible TLS have been reported in patients treated with trastuzumab. Patients with significant tumor burden (e.g. bulky metastases) may be at a higher risk. Patients could present with hyperuricemia, hyperphosphatemia, and acute renal failure which may represent possible TLS. Providers should consider additional monitoring and/or treatment as clinically indicated.
-
7 DRUG INTERACTIONSAnthracyclines - Patients who receive anthracycline after stopping HERCEPTIN HYLECTA may be at increased risk of cardiac dysfunction because of HERCEPTIN HYLECTA's estimated long washout period ...Close
Anthracyclines
Patients who receive anthracycline after stopping HERCEPTIN HYLECTA may be at increased risk of cardiac dysfunction because of HERCEPTIN HYLECTA's estimated long washout period [see Clinical Pharmacology (12.3)]. If possible, avoid anthracycline-based therapy for up to 7 months after stopping HERCEPTIN HYLECTA. If anthracyclines are used, closely monitor the patient's cardiac function.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Pharmacovigilance Program - There is a pregnancy pharmacovigilance program for HERCEPTIN HYLECTA. If HERCEPTIN HYLECTA is administered during pregnancy, or if a ...
8.1 Pregnancy
Pregnancy Pharmacovigilance Program
There is a pregnancy pharmacovigilance program for HERCEPTIN HYLECTA. If HERCEPTIN HYLECTA is administered during pregnancy, or if a patient becomes pregnant while receiving HERCEPTIN HYLECTA or within 7 months following the last dose of HERCEPTIN HYLECTA, health care providers and patients should immediately report HERCEPTIN HYLECTA exposure to Genentech at 1-888-835-2555.
Risk Summary
HERCEPTIN HYLECTA can cause fetal harm when administered to a pregnant woman. In post-marketing reports and published literature, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence, manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death [see Data]. Apprise the patient of the potential risks to a fetus. There are clinical considerations if HERCEPTIN HYLECTA is used in a pregnant woman or if a patient becomes pregnant within 7 months following the last dose of HERCEPTIN HYLECTA [see Clinical Considerations].
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Monitor women who received HERCEPTIN HYLECTA during pregnancy or within 7 months prior to conception for oligohydramnios. If oligohydramnios occurs, perform fetal/neonatal testing that is appropriate for gestational age and consistent with community standards of care.
Data
Human Data
In post-marketing reports and published literature, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence. Fetal manifestations included pulmonary hypoplasia, skeletal abnormalities, and neonatal death. These case reports described oligohydramnios in pregnant women who received trastuzumab either alone or in combination with chemotherapy. In most reported cases, amniotic fluid index increased after use of trastuzumab was stopped. In reported cases where Herceptin therapy was resumed after amniotic index improved, oligohydramnios recurred.
Animal Data
HERCEPTIN HYLECTA for subcutaneous injection contains trastuzumab and hyaluronidase [see Description (11)].
Trastuzumab:
In studies where intravenous trastuzumab was administered to pregnant cynomolgus monkeys during the period of organogenesis at doses up to 25 mg/kg given twice weekly (up to 25 times the recommended weekly human dose of 2 mg/kg), trastuzumab crossed the placental barrier during the early (Gestation Days 20 to 50) and late (Gestation Days 120 to 150) phases of gestation. The resulting concentrations of trastuzumab in fetal serum and amniotic fluid were approximately 33% and 25%, respectively, of those present in the maternal serum but were not associated with adverse developmental effects.
Hyaluronidase:
In an embryo-fetal study, mice have been dosed daily by subcutaneous injection during the period of organogenesis with hyaluronidase (recombinant human) at dose levels up to 2,200,000 U/kg, which is >7,200 times higher than the human dose. The study found no evidence of teratogenicity. Reduced fetal weight and increased numbers of fetal resorptions were observed, with no effects found at a daily dose of 360,000 U/kg, which is >1,200 times higher than the human dose.
In a peri-and post-natal reproduction study, mice have been dosed daily by subcutaneous injection, with hyaluronidase (recombinant human) from implantation through lactation and weaning at dose levels up to 1,100,000 U/kg, which is >3,600 times higher than the human dose. The study found no adverse effects on sexual maturation, learning and memory or fertility of the offspring.
8.2 Lactation
Risk Summary
There is no information regarding the presence of trastuzumab or hyaluronidase in human milk, the effects on the breastfed infant, or the effects on milk production. Published data suggest human IgG is present in human milk but does not enter the neonatal and infant circulation in substantial amounts.
Trastuzumab was present in the milk of lactating cynomolgus monkeys but not associated with neonatal toxicity (see Data). Consider the developmental and health benefits of breastfeeding along with the mother's clinical need for HERCEPTIN HYLECTA treatment and any potential adverse effects on the breastfed child from HERCEPTIN HYLECTA or from the underlying maternal condition. This consideration should also take into account the trastuzumab wash out period of 7 months [see Clinical Pharmacology 12.3].
Data
In lactating cynomolgus monkeys, trastuzumab was present in breast milk at about 0.3% of maternal serum concentrations after pre- (beginning Gestation Day 120) and post-partum (through Post-partum Day 28) doses of 25 mg/kg administered twice weekly (25 times the recommended weekly human dose of 2 mg/kg of intravenous trastuzumab). Infant monkeys with detectable serum levels of trastuzumab did not exhibit any adverse effects on growth or development from birth to 1 month of age.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of HERCEPTIN HYLECTA.
Contraception
Females
HERCEPTIN HYLECTA can cause embryo-fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment with HERCEPTIN HYLECTA and for 7 months following the last dose of HERCEPTIN HYLECTA [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
8.4 Pediatric Use
The safety and effectiveness of HERCEPTIN HYLECTA in pediatric patients have not been established.
Close8.5 Geriatric Use
Of the total number of patients in the HannaH and SafeHER studies treated with HERCEPTIN HYLECTA, 19% were 65 and over, while 4.7% were 75 and over.
In patients receiving intravenous trastuzumab, the risk of cardiac dysfunction was increased in geriatric patients as compared to younger patients, in both those receiving treatment for adjuvant therapy or metastatic disease. Other differences in safety or effectiveness were not observed between elderly patients and younger patients.
-
11 DESCRIPTIONHERCEPTIN HYLECTA is a combination of trastuzumab and hyaluronidase. Trastuzumab is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain ...
HERCEPTIN HYLECTA is a combination of trastuzumab and hyaluronidase. Trastuzumab is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2. Trastuzumab is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary) culture. Trastuzumab has a molecular weight of approximately 148 kDa.
Hyaluronidase (recombinant human) is an endoglycosidase used to increase the dispersion and absorption of co-administered drugs when administered subcutaneously. It is a glycosylated single-chain protein produced by mammalian (Chinese Hamster Ovary) cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (PH20). Hyaluronidase (recombinant human) has a molecular weight of approximately 61 kDa.
HERCEPTIN HYLECTA (trastuzumab and hyaluronidase) injection is a sterile, preservative-free, colorless to yellowish, clear to opalescent solution supplied in single-dose vials for subcutaneous administration.
HERCEPTIN HYLECTA is supplied as 600 mg trastuzumab and 10,000 units hyaluronidase per 5 mL in single-dose vials. Each mL of solution contains trastuzumab (120 mg), hyaluronidase (2,000 units), L-histidine (0.39 mg), L-histidine hydrochloride monohydrate (3.67 mg), L-methionine (1.49 mg), polysorbate 20 (0.4 mg), α,α-trehalose dihydrate (79.45 mg), and Water for Injection.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor ...
12.1 Mechanism of Action
The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab has been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress HER2.
Trastuzumab is a mediator of antibody-dependent cellular cytotoxicity (ADCC). In vitro, trastuzumab-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not overexpress HER2.
Hyaluronan is a polysaccharide found in the extracellular matrix of the subcutaneous tissue. It is depolymerized by the naturally occurring enzyme hyaluronidase. Unlike the stable structural components of the interstitial matrix, hyaluronan has a half-life of approximately 0.5 days. Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan. In the doses administered, hyaluronidase in HERCEPTIN HYLECTA acts transiently and locally.
The effects of hyaluronidase are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
Hyaluronidase has been shown to increase the absorption rate of a trastuzumab product into the systemic circulation when given in the subcutis of Göttingen Minipigs.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effects of trastuzumab on electrocardiographic (ECG) endpoints, including QTc interval duration, were evaluated in patients with HER2-positive solid tumors. Trastuzumab had no clinically relevant effect on the QTc interval duration and there was no apparent relationship between serum trastuzumab concentrations and change in QTcF interval duration in patients with HER2-positive solid tumors.
12.3 Pharmacokinetics
Trastuzumab exposure following subcutaneous administration of HERCEPTIN HYLECTA 600 mg every 3 weeks as compared to intravenous trastuzumab 8 mg/kg loading dose, 6 mg/kg maintenance every 3 weeks in the HannaH Study is shown in Table 6. The pharmacokinetic (PK) results for the co-primary endpoint, Ctrough pre-dose Cycle 8, showed non-inferiority of HERCEPTIN HYLECTA (78.7 mcg/mL) compared to intravenous trastuzumab (57.8 mcg/mL), with a geometric mean ratio of 1.3 (90% CI: 1.2–1.4).
A population PK model with parallel linear and nonlinear elimination from the central compartment was constructed using pooled HERCEPTIN HYLECTA and intravenous trastuzumab pharmacokinetic (PK) data from HannaH to describe the observed trastuzumab PK concentrations following HERCEPTIN HYLECTA subcutaneous administration and intravenous trastuzumab administration. Population PK predicted trastuzumab exposure are shown in Table 6.
Following subcutaneous administration of HERCEPTIN HYLECTA, trastuzumab concentrations were approximately at steady-state after the Cycle 7 dose with < 15% increase in concentration up to Cycle 13. The mean Ctrough at the pre-dose Cycle 18 in HERCEPTIN HYLECTA arm is similar to that of Cycle 13, suggesting no further increase after Cycle 13. The mean Cmax was 32% lower, and the mean AUC0-21 days following the Cycle 7 dose and Cycle 12 dose was approximately 10% and 20% higher, respectively, in the HERCEPTIN HYLECTA arm than in the intravenous trastuzumab arm.
Table 6 Trastuzumab Exposure (median with 5th-95th Percentiles) following Subcutaneous Administration of HERCEPTIN HYLECTA or Intravenous Trastuzumab Trastuzumab Exposure HERCEPTIN HYLECTA Intravenous Trastuzumab Ctrough (mcg/mL) Cycle 1 28.2 (14.8–40.9) 29.4 (5.8–59.5) Cycle 7 75.0 (35.1–123) 47.4 (5–114.7) Cmax (mcg/mL) Cycle 1 79.3 (56.1–109) 178 (117–291) Cycle 7 149 (86.1–214) 179 (107–309) AUC0-21 days (mcg/mL∙day) Cycle 1 1065 (718–1504) 1373 (736–2245) Cycle 7 2337 (1258–3478) 1794 (673–3618) General PK parameters of trastuzumab following subcutaneous administration of HERCEPTIN HYLECTA are shown in Table 7. Trastuzumab is estimated to reach concentrations that are < 1 mcg/mL by 7 months in at least 95% patients.
Table 7 PK parameters of Trastuzumab following Subcutaneous Administration of HERCEPTIN HYLECTA* Absorption Absolute Bioavailability 0.77 (13) First-order absorption rate, ka (day-1) 0.4 (2.92)† Tmax (day) 3 (1-14)‡ Distribution Volume of Central Compartment (L) 2.9 (19.1) Elimination Linear Elimination Clearance (L/day) 0.11 (30) Non-linear Elimination Vmax (mg/day) 11.9 (19.9)† Non-linear Elimination Km (mg/L) 33.9 (38.6)† Specific Populations
Body weight showed a statistically significant influence on PK. In patients with a body weight < 51 kg, mean steady state AUC of trastuzumab was about 80% higher after HERCEPTIN HYLECTA than after intravenous trastuzumab treatment, whereas in the highest BW group (> 90 kg) AUC was 20% lower after HERCEPTIN HYLECTA than after intravenous trastuzumab treatment. However, no body weight based dose adjustments are needed, as the exposure changes are not considered clinically relevant.
Drug Interaction Studies
There have been no formal drug interaction studies performed with trastuzumab in humans. Clinically significant interactions between trastuzumab and concomitant medications used in clinical trials have not been observed.
Paclitaxel and doxorubicin: Concentrations of paclitaxel and doxorubicin and their major metabolites (i.e., 6-α hydroxyl-paclitaxel [POH], and doxorubicinol [DOL], respectively) were not altered in the presence of trastuzumab when used as combination therapy in clinical trials. Trastuzumab concentrations were not altered as part of this combination therapy.
Close12.6 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to HERCEPTIN HYLECTA and intravenous trastuzumab in the study described below with the incidence of antibodies in other studies or to other products may be misleading.
In the HannaH study, at a median follow-up exceeding 60 months, the incidence of treatment-induced/enhanced anti-trastuzumab antibodies was 10% (30/296) in patients treated with intravenous trastuzumab and 16% (47/295) in patients receiving HERCEPTIN HYLECTA. Neutralizing anti-trastuzumab antibodies were detected in post-baseline samples in 2/30 patients in the intravenous trastuzumab arm and 3/47 patients in the HERCEPTIN HYLECTA arm. The incidence of treatment-induced/enhanced anti-recombinant human hyaluronidase antibodies was 21% (62/295) in the HERCEPTIN HYLECTA arm. None of the patients who tested positive for anti-recombinant human hyaluronidase antibodies tested positive for neutralizing antibodies.
The clinical relevance of the development of anti-trastuzumab or anti-recombinant human hyaluronidase antibodies after treatment with HERCEPTIN HYLECTA is not known.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - HERCEPTIN HYLECTA contains trastuzumab and hyaluronidase. Trastuzumab has not been tested for carcinogenicity potential. No evidence of ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
HERCEPTIN HYLECTA contains trastuzumab and hyaluronidase.
Trastuzumab has not been tested for carcinogenicity potential.
No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte mutagenicity assays at concentrations of up to 5000 mcg/mL. In an in vivo micronucleus assay, no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg of trastuzumab.
A fertility study was conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg of intravenous trastuzumab and has revealed no evidence of impaired fertility, as measured by menstrual cycle duration and female sex hormone levels.
Hyaluronidases are found in most tissues of the body. Long-term animal studies have not been performed to assess the carcinogenic or mutagenic potential of hyaluronidase. In addition, when hyaluronidase (recombinant human) was administered to cynomolgus monkeys for 39 weeks at dose levels up to 220,000 U/kg, which is > 670 times higher than the human dose, no evidence of toxicity to the male or female reproductive system was found through periodic monitoring of in-life parameters, e.g., semen analyses, hormone levels, menstrual cycles, and also from gross pathology, histopathology and organ weight data.
-
14 CLINICAL STUDIESThe comparability between HERCEPTIN HYLECTA administered subcutaneously and intravenous trastuzumab was established in the HannaH study. The HannaH study was conducted in patients with HER2 ...
The comparability between HERCEPTIN HYLECTA administered subcutaneously and intravenous trastuzumab was established in the HannaH study. The HannaH study was conducted in patients with HER2 overexpressing breast cancer in the neoadjuvant and adjuvant settings with co-primary endpoints of pathological complete response (pCR) and the PK endpoint of Ctrough at cycle 7 [see Clinical Pharmacology (12.3)].
14.1 Adjuvant Breast Cancer
HERCEPTIN HYLECTA
HannaH
The HannaH study (NCT00950300) was a randomized, multicenter, open-label, clinical trial in 596 patients with HER2-positive operable or locally advanced breast cancer (LABC), including inflammatory breast cancer. HER2-positivity was defined as IHC 3+ or ISH+. Patients were randomized to receive 8 cycles of either HERCEPTIN HYLECTA or intravenous trastuzumab concurrently with chemotherapy (docetaxel followed by 5FU, epirubicin and cyclophosphamide), followed by surgery and continued therapy with HERCEPTIN HYLECTA or intravenous trastuzumab as treated prior to surgery, for an additional 10 cycles, to complete 18 cycles of therapy. HannaH was designed to demonstrate non-inferiority of treatment with HERCEPTIN HYLECTA versus intravenous trastuzumab based on co-primary PK and efficacy outcomes (trastuzumab Ctrough at pre-dose Cycle 8, and pCR rate at definitive surgery, respectively) [see Clinical Pharmacology 12.3]. EFS and OS were among other outcomes evaluated in this study. The majority of patients were white (69%) and the median age was 50 years (range: 24-81).
The analysis of the efficacy co-primary outcome, pCR, defined as absence of invasive neoplastic cells in the breast, resulted in rates of 45.4% (95% CI: 39.2, 51.7) in the HERCEPTIN HYLECTA arm and 40.7% (95% CI: 34.7, 46.9) in the intravenous trastuzumab arm.
Table 8: Summary of Pathological Complete Response (pCR) (HannaH) HERCEPTIN HYLECTA
(n=260)Intravenous Trastuzumab
(n=263)pCR (absence of invasive neoplastic cells in breast [ypT0/is]) 118 (45.4%) 107 (40.7%) Exact 95% CI for pCR Rate* (39.2; 51.7) (34.7; 46.9) Difference in pCR (SC minus IV arm) 4.70 95% CI for Difference in pCR† (-4.0; 13.4) With a median follow-up exceeding 70 months, no difference in EFS and OS was observed in the final analysis between patients who received intravenous trastuzumab and those who received HERCEPTIN HYLECTA.
SafeHER
The SafeHER study (NCT01566721) was a prospective, two-cohort, non-randomized, multinational, open-label study designed to assess the overall safety and tolerability of HERCEPTIN HYLECTA with chemotherapy in 1864 patients with HER2-positive breast cancer. The secondary objectives include the evaluation of DFS and OS. HER2-positivity was defined as IHC 3+ or ISH+. Patients received a fixed dose of 600 mg HERCEPTIN HYLECTA every 3 weeks for a total of 18 cycles throughout the study. HERCEPTIN HYLECTA treatment was initiated either sequentially with chemotherapy, concurrently with chemotherapy, or without adjuvant chemotherapy, or in combination with neoadjuvant chemotherapy followed by trastuzumab therapy. The majority of treated patients were white (76%) and the median age was 54 years (range: 20-88).
In the primary safety analysis (median follow-up 23.7 months), no new safety signals were identified for HERCEPTIN HYLECTA. Safety and tolerability results, including in lower weight patients, were consistent with the known safety profile for HERCEPTIN HYLECTA and intravenous trastuzumab.
In the ITT population (n=1867), 126 patients (7%) had a DFS event (recurrence, contralateral invasive breast cancer or death) and 28 patients (1.5%) had an OS event at the time of clinical cut-off.
Intravenous Trastuzumab
The safety and efficacy of intravenous trastuzumab in women receiving adjuvant chemotherapy for HER2 overexpressing breast cancer were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies NSABP B31 and NCCTG N9831) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (HERA Study) with a total of 3386 women at definitive DFS analysis for 1-year intravenous trastuzumab treatment versus observation, and a fourth randomized, open-label clinical trial with a total of 3222 patients (Study BCIRG006).
Studies NSABP B31 and NCCTG N9831
In Studies NSABP B31 and NCCTG N9831, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH). HER2 testing was verified by a central laboratory prior to randomization (Study NCCTG N9831) or was required to be performed at a reference laboratory (Study NSABP B31). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or left ventricular ejection fraction findings or uncontrolled hypertension (diastolic > 100 mm Hg or systolic > 200 mm Hg) were not eligible.
Patients were randomized (1:1) to receive doxorubicin and cyclophosphamide followed by paclitaxel (AC→paclitaxel) alone or paclitaxel plus intravenous trastuzumab (AC→paclitaxel + intravenous trastuzumab). In both trials, patients received four 21-day cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2. Paclitaxel was administered either weekly (80 mg/m2) or every 3 weeks (175 mg/m2) for a total of 12 weeks in Study NSABP B31; paclitaxel was administered only by the weekly schedule in Study NCCTG N9831. Intravenous trastuzumab was administered at 4 mg/kg on the day of initiation of paclitaxel and then at a dose of 2 mg/kg weekly for a total of 52 weeks. Intravenous trastuzumab treatment was permanently discontinued in patients who developed congestive heart failure, or persistent/recurrent LVEF decline [see Dosage and Administration (2.3)]. Radiation therapy, if administered, was initiated after the completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. The major efficacy outcome of the combined efficacy analysis was DFS, defined as the time from randomization to recurrence, occurrence of contralateral breast cancer, other second primary cancer, or death. An additional efficacy outcome measure was OS.
A total of 3752 patients were included in the joint efficacy analysis of DFS following a median follow-up of 2.0 years in the AC→paclitaxel + intravenous trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC→paclitaxel + intravenous trastuzumab arm. The data from both arms in Study NSABP B31 and two of the three study arms in Study NCCTG N9831 were pooled for efficacy analyses. The patients included in the DFS analysis had a median age of 49 years (range, 22–80 years; 6% > 65 years), 84% were White, 7% Black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors.
HERA Study
In the HERA Study, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of congestive heart failure or LVEF < 55%, uncontrolled arrhythmias, angina requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on ECG, poorly controlled hypertension (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible.
Patients were randomized (1:1:1) upon completion of definitive surgery, and at least 4 cycles of chemotherapy to receive no additional treatment, or 1 year of intravenous trastuzumab treatment or 2 years of intravenous trastuzumab treatment. Patients undergoing a lumpectomy had also completed standard radiotherapy. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Intravenous trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every 3 weeks. The major efficacy outcome measure was DFS, defined as in Studies NSABP B31 and NCCTG N9831.
HERA was designed to compare 1 and 2 years of three-weekly intravenous trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). A protocol specified interim efficacy analysis comparing one-year intravenous trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the intravenous trastuzumab. Among the 3386 patients randomized to the observation (n = 1693) and intravenous trastuzumab one-year (n = 1693) treatment arms, the median age was 49 years (range 21–80), 83% were White, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with node-negative disease had high-risk features: among the 1098 patients with node-negative disease, 49% (543) were ER– and PgR–, and 47% (512) were ER+ and/or PgR+ and had at least one of the following high-risk features: pathological tumor size > 2 cm, Grade 2–3, or age < 35 years. Prior to randomization, 94% of patients had received anthracycline-based chemotherapy regimens.
After the DFS results comparing observation to one-year intravenous trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of intravenous trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending intravenous trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years intravenous trastuzumab versus one-year intravenous trastuzumab treatment in the ITT population for DFS = 0.99 (95% CI: 0.87, 1.13), p = 0.90 and OS = 0.98 (0.83, 1.15); p = 0.78].
BCIRG006 Study
In the BCIRG006 Study, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or node-negative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of CHF, myocardial infarction, Grade 3 or 4 cardiac arrhythmia, angina requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mm Hg), any T4 or N2, or known N3 or M1 breast cancer were not eligible.
Patients were randomized (1:1:1) to receive doxorubicin and cyclophosphamide followed by docetaxel (AC-T), doxorubicin and cyclophosphamide followed by docetaxel plus intravenous trastuzumab (AC-TH), or docetaxel and carboplatin plus intravenous trastuzumab (TCH). In both the AC-T and AC-TH arms, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 were administered every 3 weeks for four cycles; docetaxel 100 mg/m2 was administered every 3 weeks for four cycles. In the TCH arm, docetaxel 75 mg/m2 and carboplatin (at a target AUC of 6 mg/mL/min as a 30- to 60-minute infusion) were administered every 3 weeks for six cycles. Intravenous trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as monotherapy for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. DFS was the major efficacy outcome measure.
Among 3222 patients, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for breast cancer.
The results for DFS for the integrated analysis of Studies NSABP B31 and NCCTG N9831, HERA, and BCIRG006 and OS results for the integrated analysis of Studies NSABP B31 and NCCTG N9831, and HERA are presented in Table 9. For Studies NSABP B31 and NCCTG N9831, the duration of DFS following a median follow-up of 2.0 years in the AC→TH arm is presented in Figure 1, and the duration of OS after a median follow-up of 8.3 years in the AC→TH arm is presented in Figure 2. The duration of DFS for BCIRG006 is presented in Figure 3. For Studies NSABP B31 and NCCTG N9831, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow-up [AC→TH], the survival rate was estimated to be 86.9% in the AC→TH arm and 79.4% in the AC→T arm. The final OS analysis results from Studies NSABP B31 and NCCTG N9831 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n = 2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n = 1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n = 2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n = 1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤ 2 cm (n = 1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size > 2 cm (n = 2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80).
Table 9 Efficacy Results from Adjuvant Treatment of Breast Cancer (Studies NSABP B31, NCCTG N9831, HERA, and BCIRG006) DFS events DFS Hazard ratio
(95% CI)
p-valueDeaths
(OS events)OS Hazard ratio
p-valueCI = confidence interval. - *
- Studies NSABP B31 and NCCTG N9831 regimens: doxorubicin and cyclophosphamide followed by paclitaxel (AC→T) or paclitaxel plus intravenous trastuzumab (AC→TH).
- †
- Efficacy evaluable population, for the primary DFS analysis, following a median follow-up of 2.0 years in the AC→TH arm.
- ‡
- Efficacy evaluable population, for the final OS analysis, following 707 deaths (8.3 years of median follow-up in the AC→TH arm).
- §
- Hazard ratio estimated by Cox regression stratified by clinical trial, intended paclitaxel schedule, number of positive nodes, and hormone receptor status.
- ¶
- stratified log-rank test.
- #
- At definitive DFS analysis with median duration of follow-up of 12.6 months in the one-year intravenous trastuzumab treatment arm.
- Þ
- log-rank test.
- ß
- NS = non-significant.
- à
- BCIRG006 regimens: doxorubicin and cyclophosphamide followed by docetaxel (AC→T) or docetaxel plus intravenous trastuzumab (AC→TH); docetaxel and carboplatin plus intravenous trastuzumab (TCH).
- è
- A two-sided alpha level of 0.025 for each comparison.
Studies NSABP B31 and NCCTG N9831* AC→TH
(n = 1872)†
(n = 2031)‡133† 0.48†,§
(0.39, 0.59)
p< 0.0001¶289‡ 0.64‡,§
(0.55, 0.74)
p< 0.0001¶AC→T
(n = 1880)†
(n = 2032)‡261† 418‡ HERA# Chemo→
Intravenous trastuzumab
(n = 1693)127 0.54
(0.44, 0.67)
p< 0.0001Þ31 0.75
p = NSßChemo→
Observation
(n = 1693)219 40 BCIRG006à TCH
(n = 1075)134 0.67
(0.54 – 0.84)
p=0.0006¶,è56 AC→TH
(n = 1074)121 0.60
(0.48 – 0.76)
p< 0.0001¶,è49 AC→T
(n = 1073)180 80 Figure 1 Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (Studies NSABP B31 and NCCTG N9831) 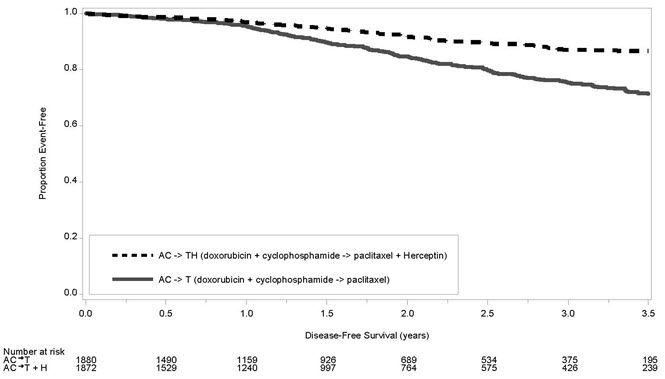
Figure 2 Duration of Overall Survival in Patients with Adjuvant Treatment of Breast Cancer (Studies NSABP B31 and NCCTG N9831) 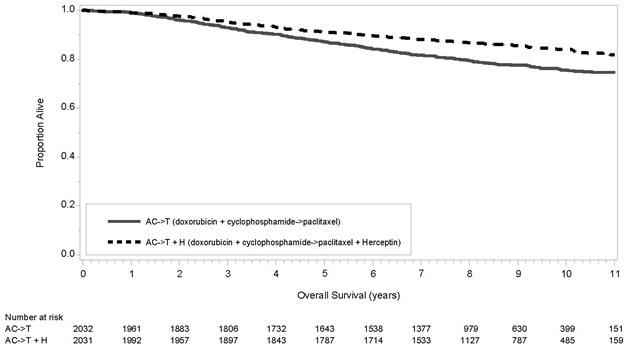
Figure 3 Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (BCIRG006) 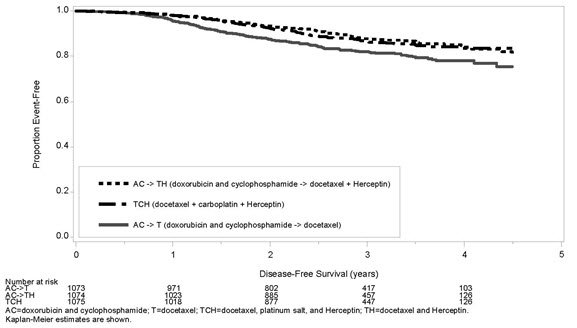
Exploratory analyses of DFS as a function of HER2 overexpression or gene amplification were conducted for patients in Study NCCTG N9831 and HERA, where central laboratory testing data were available. The results are shown in Table 10. The number of events in Study NCCTG N9831 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in HERA was adequate to demonstrate significant effects on DFS in the IHC 3+/FISH unknown and the FISH+/IHC unknown subgroups.
Table 10 Treatment Outcomes in Study NCCTG N9831 and HERA for Patients with HER2 Overexpression or Amplification Study NCCTG N9831 HERA* HER2 Assay Result† Number of Patients Hazard Ratio DFS (95% CI) Number of Patients Hazard Ratio DFS (95% CI) IHC 3+ FISH (+) 1170 0.42
(0.27, 0.64)91 0.56
(0.13, 2.50)FISH (−) 51 0.71
(0.04, 11.79)8 — FISH Unknown 51 0.69
(0.09, 5.14)2258 0.53
(0.41, 0.69)IHC < 3+ / FISH (+) 174 1.01
(0.18, 5.65)299‡ 0.53
(0.20, 1.42)IHC unknown / FISH (+) — — 724 0.59
(0.38, 0.93)14.2 Metastatic Breast Cancer
Intravenous Trastuzumab
The safety and efficacy of intravenous trastuzumab in the treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (H0648g, n=469 patients) and an open-label single agent clinical trial (H0649g, n=222 patients). Both trials studied patients with metastatic breast cancer whose tumors overexpress the HER2 protein. Patients were eligible if they had level 2 or 3 overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab.
Previously Untreated Metastatic Breast Cancer (H0648g)
H0648g was a multicenter, randomized, open-label clinical trial conducted in 469 women with metastatic breast cancer who had not been previously treated with chemotherapy for metastatic disease. Patients were randomized to receive chemotherapy alone or in combination with trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of intravenous trastuzumab at 2 mg/kg. For those who had received prior anthracycline therapy in the adjuvant setting, chemotherapy consisted of paclitaxel (175 mg/m2 over 3 hours every 21 days for at least six cycles); for all other patients, chemotherapy consisted of anthracycline plus cyclophosphamide (AC: doxorubicin 60 mg/m2 or epirubicin 75 mg/m2 plus 600 mg/m2 cyclophosphamide every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received intravenous trastuzumab at the time of disease progression as part of a separate extension study.
Based upon the determination by an Independent Response Evaluation Committee, the patients randomized to intravenous trastuzumab and chemotherapy experienced a significantly longer time to disease progression (TTP), a higher overall response rate (ORR), and a longer median duration of response as compared with patients randomized to chemotherapy alone. Patients randomized to intravenous trastuzumab and chemotherapy also had a longer median overall survival (OS) (see Table 11). These treatment effects were observed both in patients who received intravenous trastuzumab plus paclitaxel and in those who received intravenous trastuzumab plus AC; however the magnitude of the effects was greater in the paclitaxel subgroup.
Table 11: H0648g: Efficacy Results in First-Line Treatment for Metastatic Breast Cancer Combined Results Paclitaxel Subgroup AC Subgroup Intravenous Trastuzumab + All Chemotherapy
(n=235)All Chemotherapy
(n=234)Intravenous Trastuzumab + Paclitaxel
(n=92)Paclitaxel
(n=96)Intravenous Trastuzumab + AC*
(n=143)AC
(n=138)Time to Disease Progression (TTP) Median (months)†,‡ 7.2 4.5 6.7 2.5 7.6 5.7 95% CI 7, 8 4, 5 5, 10 2, 4 7, 9 5, 7 p-value§ < 0.0001 < 0.0001 0.002 Overall Response Rate (ORR)† Events (n) 45 29 38 15 50 38 95% CI 39, 51 23, 35 28, 48 8, 22 42, 58 30, 46 p-value¶ < 0.001 < 0.001 0.10 Duration of Response (DoR) Median (months)†,‡ 8.3 5.8 8.3 4.3 8.4 6.4 25%, 75% Quartile 6, 15 4, 8 5, 11 4, 7 6, 15 4, 8 Overall Survival (OS) Median (months)‡ 25.1 20.3 22.1 18.4 26.8 21.4 95% CI 22, 30 17, 24 17, 29 13, 24 23, 33 18, 27 p-value§ 0.05 0.17 0.16 Data from H0648g suggest that the beneficial treatment effects were largely limited to patients with the highest level of HER2 protein overexpression (3+) (see Table 12).
Table 12: Treatment Effects in H0648g as a Function of HER2 Overexpression or Amplification HER2 Assay Result Number of Patients
(N)Relative Risk * for Time to Disease Progression
(95% CI)Relative Risk * for Mortality
(95% CI)CTA 2+ or 3+ 469 0.49 (0.40, 0.61) 0.80 (0.64, 1.00) FISH (+) † 325 0.44 (0.34, 0.57) 0.70 (0.53, 0.91) FISH (−) † 126 0.62 (0.42, 0.94) 1.06 (0.70, 1.63) CTA 2+ 120 0.76 (0.50, 1.15) 1.26 (0.82, 1.94) FISH (+) 32 0.54 (0.21, 1.35) 1.31 (0.53, 3.27) FISH (−) 83 0.77 (0.48, 1.25) 1.11 (0.68, 1.82) CTA 3+ 349 0.42 (0.33, 0.54) 0.70 (0.51, 0.90) FISH (+) 293 0.42 (0.32, 0.55) 0.67 (0.51, 0.89) FISH (−) 43 0.43 (0.20, 0.94) 0.88 (0.39, 1.98) Previously Treated Metastatic Breast Cancer (Study H0649g)
Intravenous trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (Study H0649g) in patients with HER2 overexpressing MBC who had relapsed following one or two prior chemotherapy regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior chemotherapy regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of trastuzumab at 2 mg/kg IV.
The ORR (complete response + partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes. The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%.
Close14.3 Patient Experience
The PrefHER study (NCT01401166) was a randomized, multi-center, two-arm, cross-over trial conducted in 240 patients with HER2-positive breast cancer undergoing neoadjuvant or adjuvant treatment. One hundred twenty-one patients in arm A received 4 cycles of HERCEPTIN HYLECTA followed by 4 cycles of intravenous trastuzumab and 119 patients in arm B received 4 cycles of intravenous trastuzumab followed by 4 cycles of HERCEPTIN HYLECTA. Both arms received a total of 18 cycles. After Cycle 8, 199 of 231 patients (86%) reported preferring subcutaneous administration of HERCEPTIN HYLECTA over intravenous trastuzumab and the most common reason cited was administration required less time (179/231) in the clinic. After Cycle 8, 29 out of 231 patients (13%) reported preferring intravenous trastuzumab over HERCEPTIN HYLECTA and the most common reason was fewer local injection reactions. Three out of 231 patients (1%) had no preference for the route of administration. Nine out of 240 (3.8%) withdrew from treatment prior to completion of Cycle 8 and did not complete the post-study preference questionnaire.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHERCEPTIN HYLECTA (trastuzumab and hyaluronidase-oysk) injection for subcutaneous use supplied as a sterile, preservative-free, colorless to yellowish, clear to opalescent solution in a ...
HERCEPTIN HYLECTA (trastuzumab and hyaluronidase-oysk) injection for subcutaneous use supplied as a sterile, preservative-free, colorless to yellowish, clear to opalescent solution in a single-dose vial. The following configuration is available:
Individually packaged single-dose vials:
- HERCEPTIN HYLECTA 600 mg/10,000 units (NDC: 50242-077-01) providing 600 mg trastuzumab and 10,000 units hyaluronidase per 5 mL.
CloseStore HERCEPTIN HYLECTA vials in the refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze. Do not shake. Once removed from the refrigerator, HERCEPTIN HYLECTA must be administered within 4 hours and should not be kept above 30°C (86°F).
-
17 PATIENT COUNSELING INFORMATIONCardiomyopathy - Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs ...
Cardiomyopathy
- Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, swelling of the face, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness [see Warnings and Precautions (5.1)].
Embryo-Fetal Toxicity [see Warnings and Precautions (5.2)]
- Advise pregnant women and females of reproductive potential that HERCEPTIN HYLECTA exposure during pregnancy or within 7 months prior to conception can result in fetal harm. Advise female patients to contact their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment and for 7 months following the last dose of HERCEPTIN HYLECTA [see Use in Specific Populations (8.3)].
- Advise women who are exposed to HERCEPTIN HYLECTA during pregnancy or who become pregnant within 7 months following the last dose of HERCEPTIN HYLECTA that there is a pregnancy pharmacovigilance program that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to Genentech [see Use in Specific Populations (8.1)].
CloseHypersensitivity and Administration-Related Reactions
- Advise patients to contact their healthcare provider immediately and to report any symptoms of hypersensitivity and administration-related reactions including dizziness, nausea, chills, fever, vomiting, diarrhea, urticaria, angioedema, breathing problems, or chest pain [see Warnings and Precautions (5.5)].
-
SPL UNCLASSIFIED SECTIONHERCEPTIN HYLECTA® [trastuzumab and hyaluronidase-oysk] Manufactured by: Genentech, Inc. A Member of the Roche Group - 1 DNA Way - South San Francisco, CA 94080-4990 - US License No. 1048 - HERCEPTIN ...
HERCEPTIN HYLECTA® [trastuzumab and hyaluronidase-oysk]
Manufactured by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048HERCEPTIN HYLECTA is a registered trademark of Genentech, Inc.
Close
©2024 Genentech, Inc. -
PRINCIPAL DISPLAY PANEL - 600 mg and 10,000 units/5 mL Vial CartonNDC 50242-077-01 - Herceptin Hylecta® (trastuzumab and - hyaluronidase-oysk) Injection - 600 mg and - 10,000 units/5 mL - (120 mg and 2,000 units/mL) For Subcutaneous Use Only - Single-Dose Vial - Discard ...
NDC 50242-077-01
Herceptin Hylecta®
(trastuzumab and
hyaluronidase-oysk) Injection600 mg and
10,000 units/5 mL
(120 mg and 2,000 units/mL)For Subcutaneous Use Only
Single-Dose Vial
Discard Unused Portion1 vial
Rx only
Genentech11025235
Close
-
INGREDIENTS AND APPEARANCEProduct Information
HERCEPTIN HYLECTA trastuzumab and hyaluronidase-oysk injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-077 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRASTUZUMAB (UNII: P188ANX8CK) (TRASTUZUMAB - UNII:P188ANX8CK) TRASTUZUMAB 600 mg in 5 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 10000 U in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 18.35 mg in 5 mL HISTIDINE (UNII: 4QD397987E) 1.95 mg in 5 mL TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 397.25 mg in 5 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 2 mg in 5 mL METHIONINE (UNII: AE28F7PNPL) 7.45 mg in 5 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-077-01 1 in 1 CARTON 02/28/2019 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761106 02/28/2019 Labeler - Genentech, Inc. (080129000) Registrant - Roche Singapore Technical Operations Pte. Ltd (937189173) Establishment Name Address ID/FEI Business Operations Roche Singapore Technical Operations Pte. Ltd 937189173 ANALYSIS(50242-077) , API MANUFACTURE(50242-077) Establishment Name Address ID/FEI Business Operations Roche Diagnostics 323105205 API MANUFACTURE(50242-077) , ANALYSIS(50242-077) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 315028860 ANALYSIS(50242-077) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd. 485244961 MANUFACTURE(50242-077) , ANALYSIS(50242-077) , LABEL(50242-077) , PACK(50242-077) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 ANALYSIS(50242-077)
CloseEstablishment Name Address ID/FEI Business Operations Genentech, Inc. (Oceanside) 146373191 ANALYSIS(50242-077)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
HERCEPTIN HYLECTA- trastuzumab and hyaluronidase-oysk injection, solution
Number of versions: 7
RxNorm
HERCEPTIN HYLECTA- trastuzumab and hyaluronidase-oysk injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2119714 | trastuzumab-hyaluronidase-oysk 600 MG / 10,000 UNT in 5 mL Injection | PSN |
| 2 | 2119714 | 5 ML hyaluronidase-oysk 2000 UNT/ML / trastuzumab-oysk 120 MG/ML Injection | SCD |
| 3 | 2119714 | trastuzumab-hyaluronidase-oysk 600 MG / 10,000 UNT per 5 ML Injection | SY |
| 4 | 2119714 | trastuzumab-oysk 600 MG / hyaluronidase-oysk 10,000 UNT per 5 ML Injection | SY |
| 5 | 2119719 | Herceptin Hylecta 600 MG / 10,000 UNT in 5 mL Injection | PSN |
| 6 | 2119719 | 5 ML hyaluronidase-oysk 2000 UNT/ML / trastuzumab-oysk 120 MG/ML Injection [Herceptin Hylecta] | SBD |
| 7 | 2119719 | Herceptin Hylecta 600 MG / 10,000 UNT per 5 ML Injection | SY |
| 8 | 2119719 | Herceptin Hylecta (trastuzumab-hyaluronidase-oysk 600 MG / 10,000 UNT) per 5 ML Injection | SY |
Get Label RSS Feed for this Drug
HERCEPTIN HYLECTA- trastuzumab and hyaluronidase-oysk injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=ebf30894-41cf-480c-8bc3-56f592a13813
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
HERCEPTIN HYLECTA- trastuzumab and hyaluronidase-oysk injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50242-077-01 |

