Label: ACETAMINOPHEN tablet, coated
- NDC Code(s): 72036-104-01, 72036-104-02, 72036-104-04, 72036-104-06, view more
- Packager: HARRIS TEETER
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
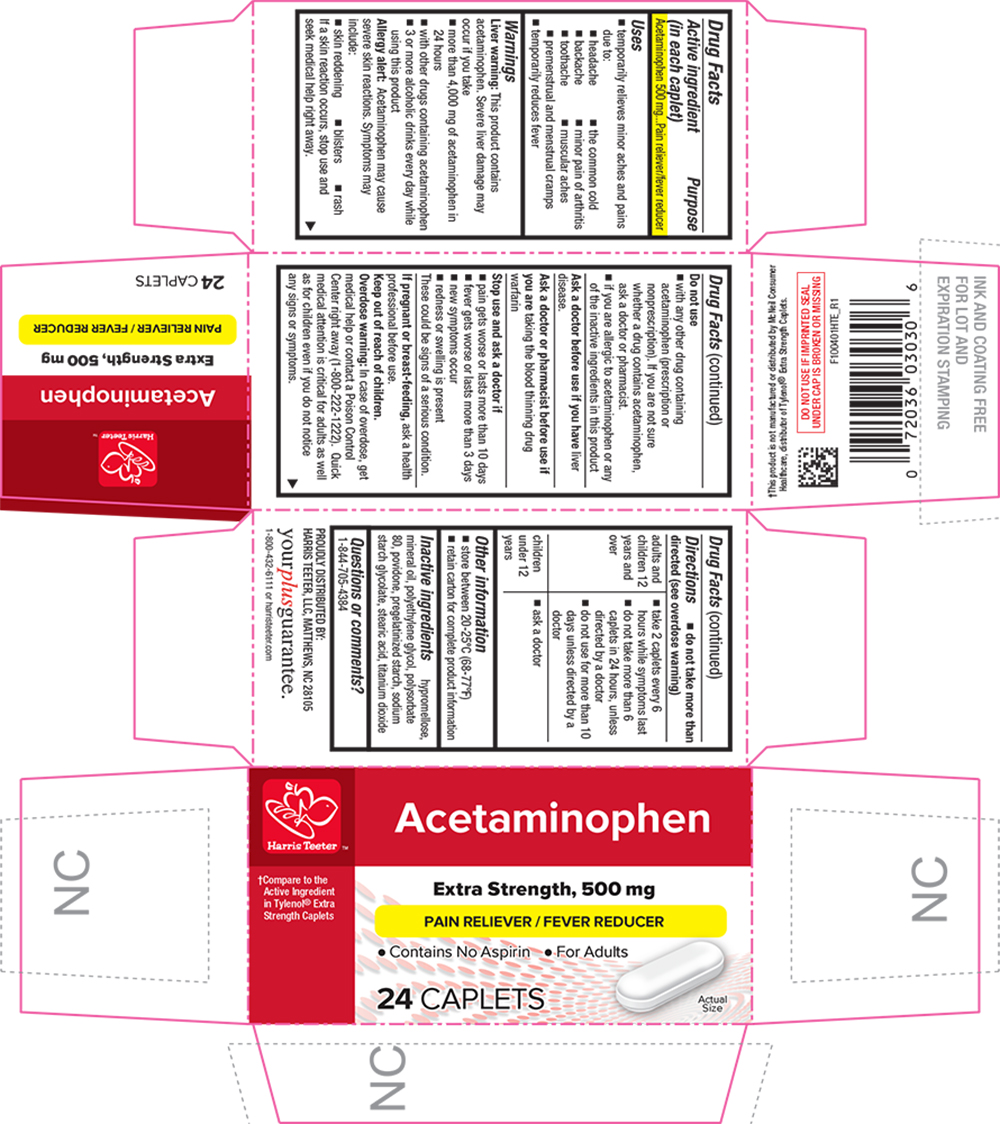
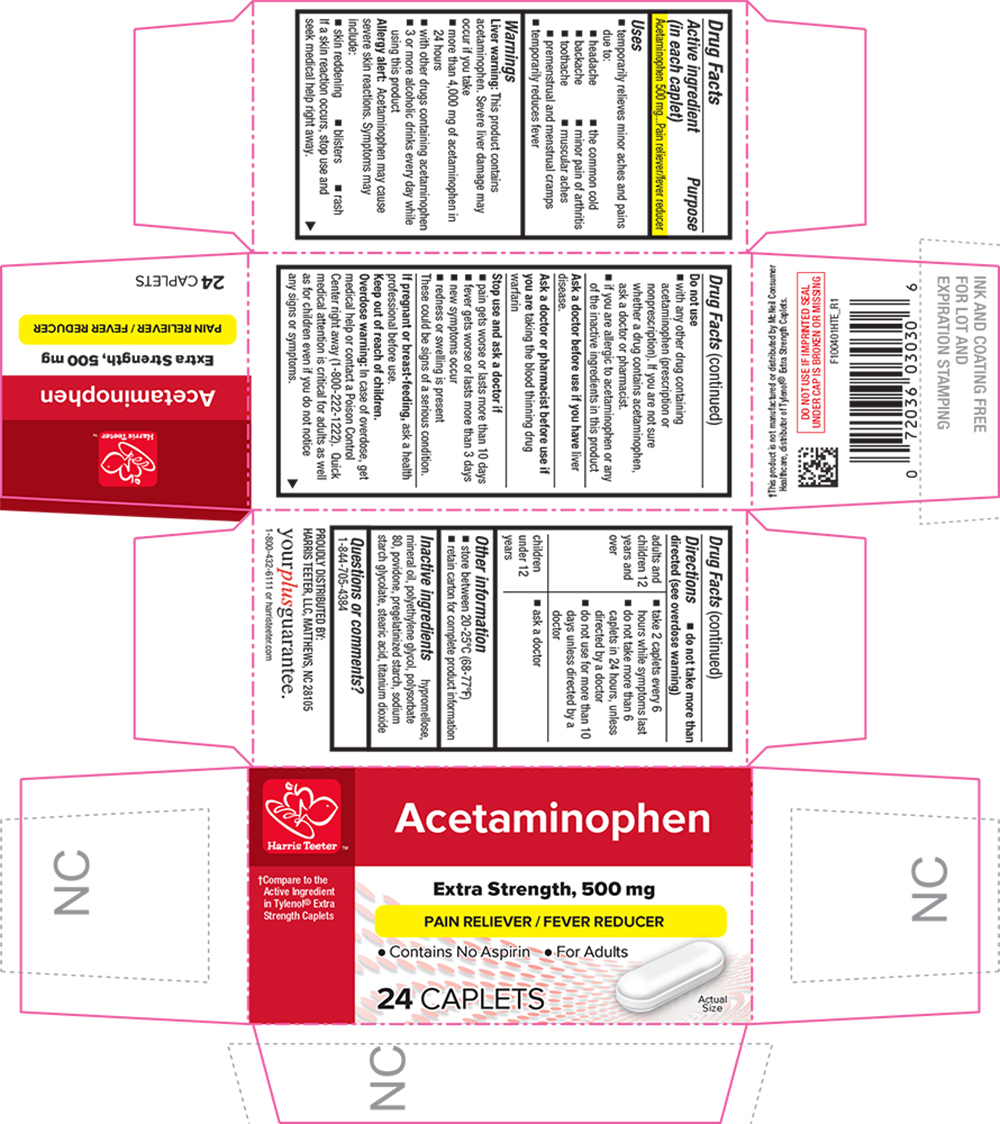
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each caplet)Acetaminophen 500 mg
-
PurposePain reliever/fever reducer
-
Usestemporarily relieves minor aches and pains due to: the common cold - headache - backache - minor pain of arthritis - toothache - muscular aches - premenstrual and menstrual ...
-
WarningsLiver warning - This product contains acetaminophen. Severe liver damage may occur if you take - more than 4,000 mg of acetaminophen in 24 hours - with other drugs containing acetaminophen - 3 or more ...
-
Directionsdo not take more than directed (see - overdose warning) adults and children 12 years and over - take 2 caplets every 6 hours while symptoms last - do not ...
-
Other informationstore between 20-25°C (68-77°F) retain carton for complete product information
-
Inactive ingredientshypromellose, mineral oil, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, stearic acid, titanium dioxide
-
Questions or comments?1-844-705-4384
-
PRINCIPAL DISPLAY PANELAcetaminophen - †Compare to the Active Ingredient in Tylenol® Extra Strength Caplets - Extra Strength, 500 mg - PAIN RELIEVER / FEVER REDUCER - Contains No Aspirin l For Adults - 24 CAPLETS - Actual ...
-
INGREDIENTS AND APPEARANCEProduct Information