Label: GRISOFULVIN suspension
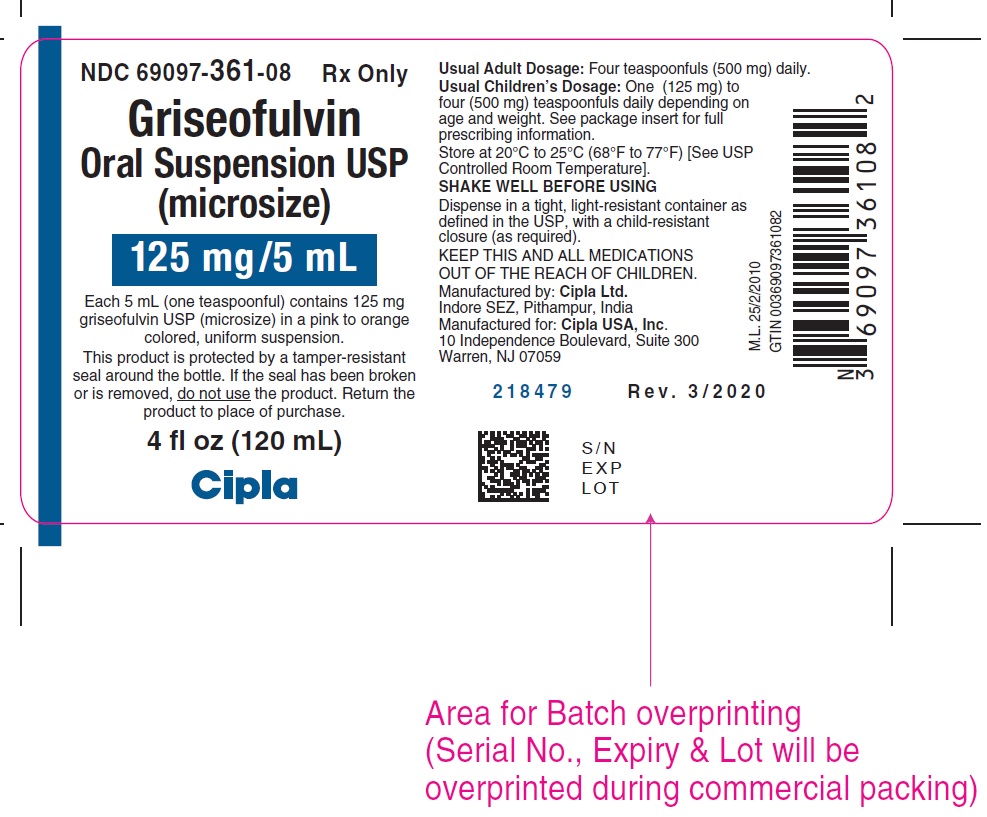
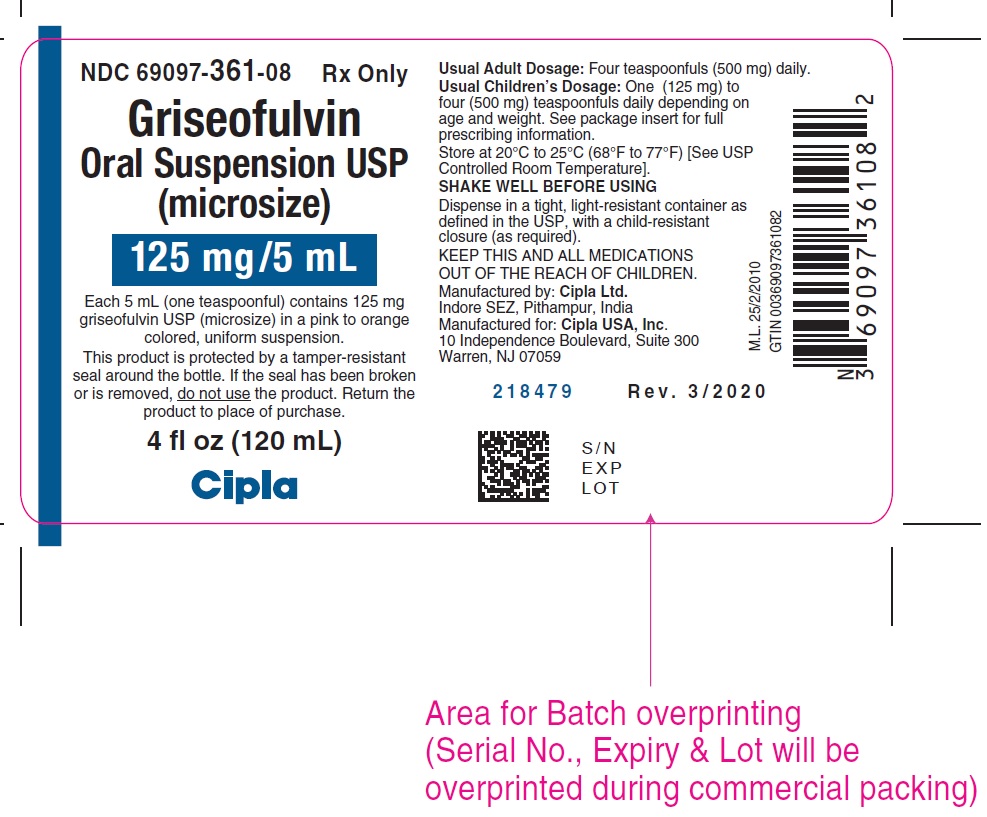
- NDC Code(s): 69097-361-08
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
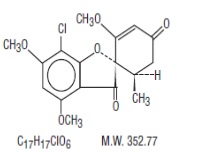
DESCRIPTIONGriseofulvin oral suspension USP (microsize) contains griseofulvin, USP (microsize) for oral administration. The active ingredient, griseofulvin, USP, is a fungistatic antibiotic, derived from a ...
-
CLINICAL PHARMACOLOGYGriseofulvin absorption from the gastrointestinal tract varies considerably among individuals mainly because of insolubility of the drug in aqueous media of the upper GI tract. Drug absorption ...
-
INDICATIONS AND USAGEGriseofulvin oral suspension is indicated for the treatment of dermatophyte infections of the skin not adequately treated by topical therapy, hair and nails, namely: Tinea corporis - Tinea ...
-
CONTRAINDICATIONSGriseofulvin is contraindicated in patients with porphyria or hepatocellular failure, and in individuals with a history of hypersensitivity to griseofulvin. Griseofulvin may cause fetal harm when ...
-
WARNINGSProphylactic Usage - Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established. Serious Skin Reactions - Severe skin reactions (e.g. Stevens-Johnson ...
-
PRECAUTIONSGeneral - Patients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic and hematopoietic ...
-
ADVERSE REACTIONSThere have been postmarketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see WARNINGS section). When adverse reactions occur, they are most commonly of ...
-
OVERDOSAGEThere is limited experience on overdose with griseofulvin. In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures as required.
-
DOSAGE AND ADMINISTRATIONAccurate diagnosis of the infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium ...
-
HOW SUPPLIEDGriseofulvin Oral Suspension USP is available as a pink to orange colored, tutti-frutti flavored, uniform suspension containing 125 mg/5 mL griseofulvin, USP (microsize) in a 4 ounce (120 mL ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-361-08 RX only - Griseofulvin - Oral Suspension USP - (microsize) 125mg/5mL - Each 5 mL (one teaspoonful) contains 125 mg - griseofulvin USP (microsize) in a pink to orange - colored ...
-
INGREDIENTS AND APPEARANCEProduct Information