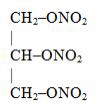Label: GONITRO- nitroglycerin powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 70007-400-01, 70007-400-12, 70007-400-36, 70007-400-96, view more - Packager: Espero Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 20, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GONITRO safely and effectively. See full prescribing information for GONITRO. GONITRO (nitroglycerin) sublingual ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
GONITRO is indicated for acute relief of an attack or prophylaxis of angina pectoris due to coronary artery disease.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - Administer one or two packets (400 mcg of nitroglycerin per packet) at the onset of an attack under the tongue. One additional packet may be administered every five ...
-
3 DOSAGE FORMS AND STRENGTHS
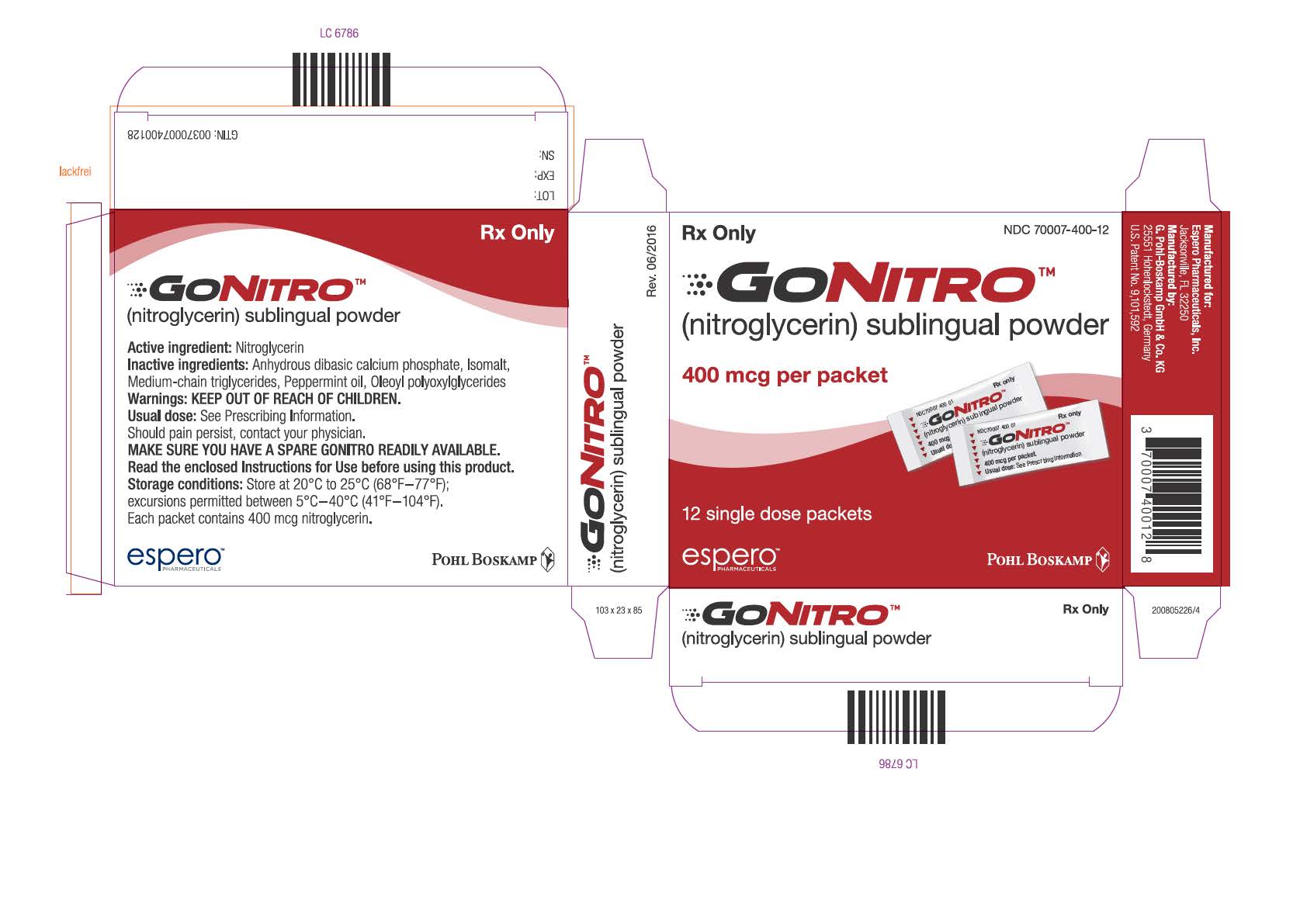
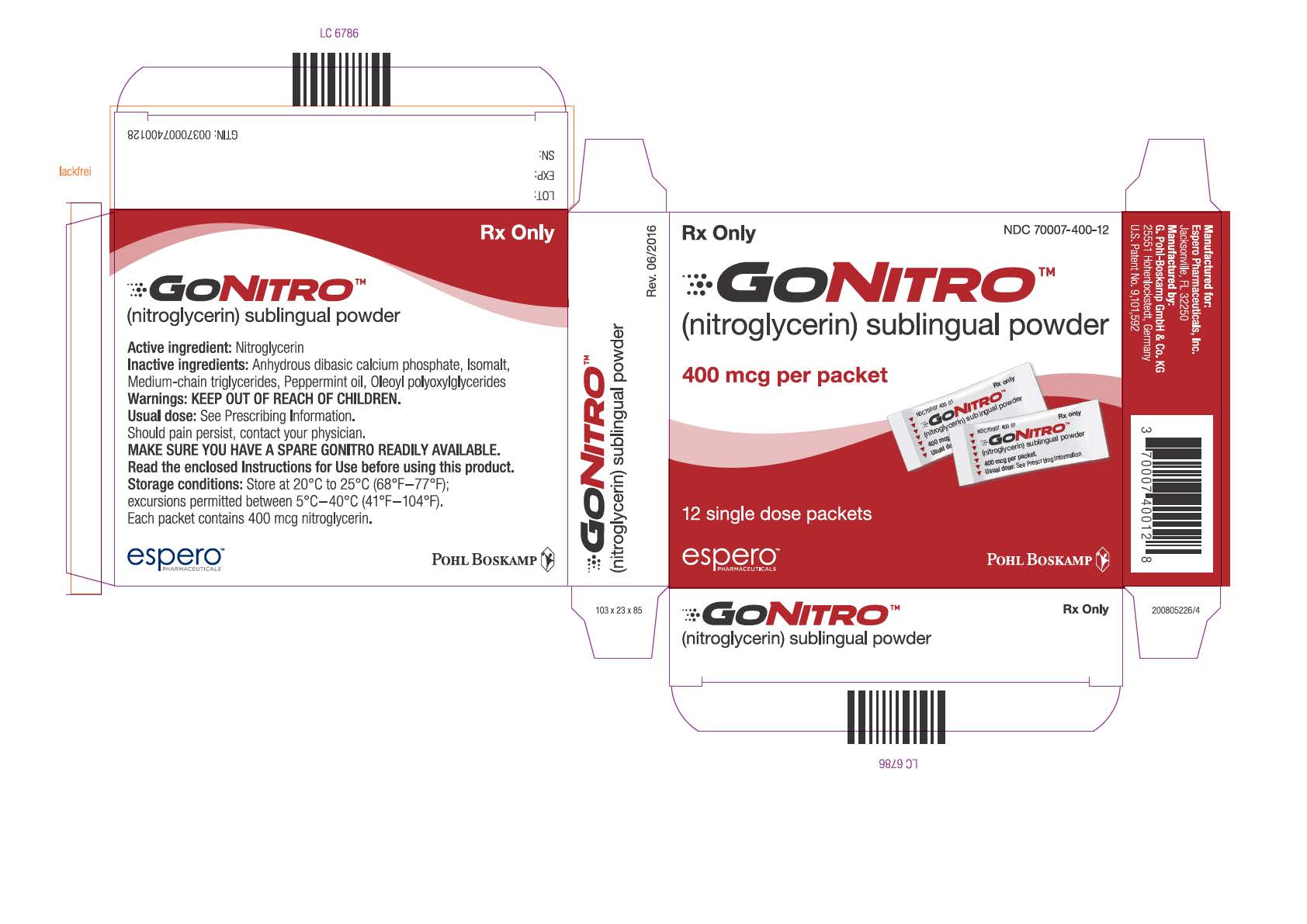
GONITRO (nitroglycerin) sublingual powder, in packets containing 400 mcg of nitroglycerin.
-
4 CONTRAINDICATIONS
4.1 PDE-5-Inhibitors and sGC-Stimulators - Do not use GONITRO in patients who are taking PDE-5-Inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil. Concomitant use can cause ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Tolerance - Excessive use may lead to the development of tolerance. Only the smallest number of doses required for effective relief of the acute angina attack should be used [see Dosage and ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 PDE-5-Inhibitors and sGC-Stimulators - GONITRO is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk summary - Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal ...
-
10 OVERDOSAGE
10.1 Signs and Symptoms, Methemoglobinemia - Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and ...
-
11 DESCRIPTION
Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3',5'-monophosphate (cyclic GMP) in ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis,Mutagenesis, Impairment of Fertility - Animal carcinogenesis studies with sublingual nitroglycerin have not been performed. Rats receiving up to 434 mg/kg/day of dietary ...
-
14 CLINICAL STUDIES
In a randomized, double-blind single-dose, 5-period cross-over study in 51 patients with exertional angina pectoris significant dose-related increases in exercise tolerance, time to onset of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each box of GONITRO (nitroglycerin) sublingual powder, contains 12, 36 or 96 packets. Each packet contains 400 mcg of nitroglycerin. GONITRO is available as: • Box of 12 packets NDC 70007-400-12 - ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved Instructions for Use. Manufactured for - Espero Pharmaceuticals, Inc. Jacksonville, FL 32250 - (800) 235-6520 - by G. Pohl-Boskamp GmbH & Co. KG - 25551 ...
-
PATIENT PACKAGE INSERTInstructions for Use - GONITRO (GO-NYE-troh) (nitroglycerin) sublingual powder - Read these Instructions for Use before you start taking GONITRO and each time you get a refill. There may be ...
-
PRINCIPAL DISPLAY PANELPrinicpal Display Panel - NDC 70007-400-12 - GONITRO™ (nitroglycerin) sublingual powder - 400 mcg per packet - 12 single dose packets - Prinicpal Display Panel - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information