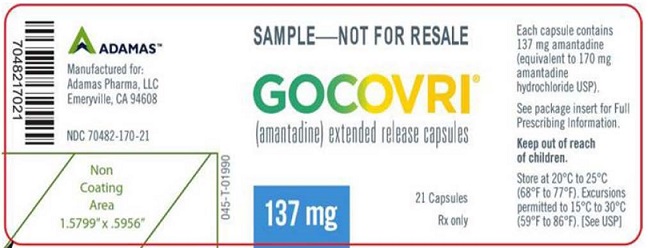
Label: GOCOVRI- amantadine capsule, coated pellets
- NDC Code(s): 70482-085-21, 70482-085-60, 70482-085-61, 70482-170-21, view more
- Packager: Adamas Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONGOCOVRI ® - These highlights do not include all the information needed to use GOCOVRI - ®safely and effectively. See full prescribing information for GOCOVRI. GOCOVRI - ®(amantadine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGOCOVRI - ®is indicated: For the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy, with or without concomitant dopaminergic medications - As ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The initial daily dosage of GOCOVRI is 137 mg, administered orally once daily at bedtime. After one week, increase to the recommended dosage of 274 mg (two 137 mg ...
-
3 DOSAGE FORMS AND STRENGTHSGOCOVRI is available as extended-release capsules for oral administration. Each capsule contains 68.5 mg or 137 mg of amantadine. The 68.5 mg capsule is a white opaque size #2 capsule, with black ...
-
4 CONTRAINDICATIONSGOCOVRI is contraindicated in patients with end-stage renal disease (i.e., creatinine clearance below 15 mL/min/1.73 m - 2) [see Clinical Pharmacology ( 12.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Falling Asleep During Activities of Daily Living and Somnolence - Patients treated for Parkinson’s disease have reported falling asleep while engaged in activities of daily living, including ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described in more detail elsewhere in the labeling: Falling Asleep During Activities of Daily Living and Somnolence - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Other Anticholinergic Drugs - Products with anticholinergic properties may potentiate the anticholinergic-like side effects of amantadine. The dose of anticholinergic drugs or of GOCOVRI ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with use of amantadine in pregnant women. Animal studies suggest a potential risk for fetal harm ...
-
10 OVERDOSAGEDeaths have been reported from overdose with amantadine. The lowest reported acute lethal dose was 1 gram of amantadine hydrochloride (equivalent to 0.8 g amantadine). Acute toxicity may be ...
-
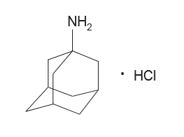
11 DESCRIPTIONGOCOVRI contains amantadine in an extended-release formulation. The active ingredient in GOCOVRI is amantadine hydrochloride. The chemical name for amantadine hydrochloride is tricyclo [3.3.1.1 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism by which amantadine exerts efficacy in the treatment of dyskinesia in patients with Parkinson’s disease or as adjunctive treatment to levodopa/carbidopa ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal studies designed to evaluate the carcinogenic potential of amantadine have not been ...
-
14 CLINICAL STUDIESOverview of Studies - The efficacy of GOCOVRI for the treatment of dyskinesia in patients with Parkinson’s disease and for the adjunctive treatment to levodopa/carbidopa in patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - GOCOVRI is supplied as extended release capsules in the following configurations: The 68.5 mg capsule is a white opaque size #2 capsule, with black printing of ‘ADAMAS’ on ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Administration - Instruct patients and caregivers that GOCOVRI capsules should be swallowed whole and can be ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - GOCOVRI (goh-KUV-ree) (amantadine) extended release capsules, for oral use - What is GOCOVRI? GOCOVRI is a prescription medicine used: for the ...
-
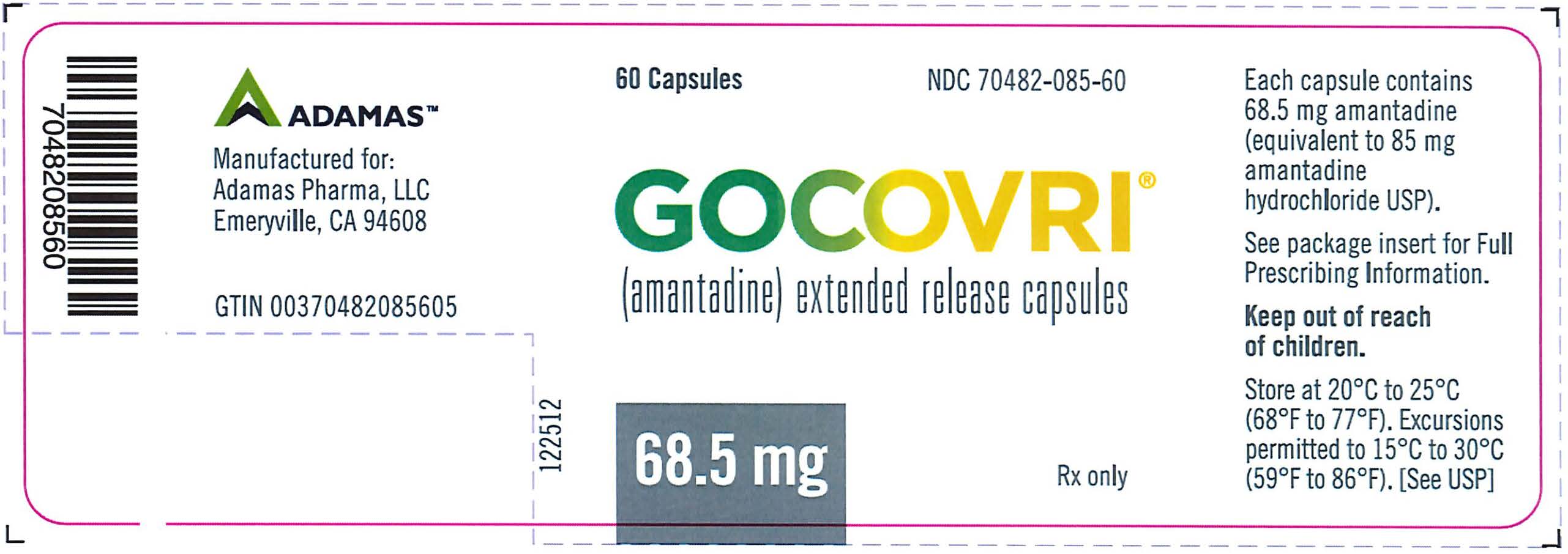
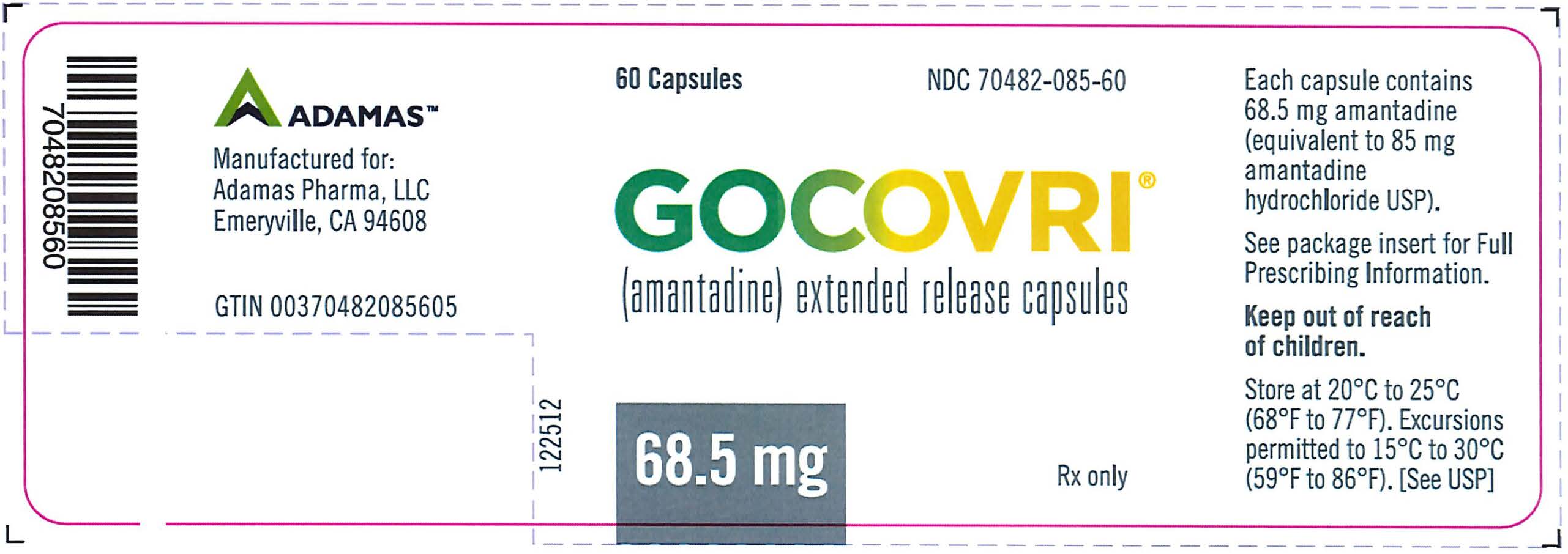
PRINCIPAL DISPLAY PANELNDC 70482-085-60 - Gocovri® 68.5 mg 60 Capsule Bottle Label
-
PRINCIPAL DISPLAY PANELNDC 70482-085-21 - Gocovri® 68.5 mg Sample Package, 21 Capsule Bottle Label
-
PRINCIPAL DISPLAY PANELNDC 70482-085-61 - Gocovri® 68.5 mg Sample Package, 60 Capsule Bottle Label
-
PRINCIPAL DISPLAY PANELNDC 70482-170-60 - Gocovri® 137 mg 60 Capsule Bottle Label
-
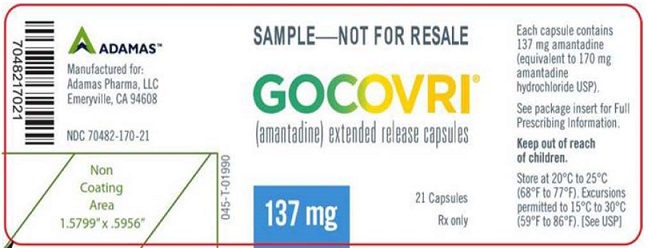
PRINCIPAL DISPLAY PANELNDC 70482-170-21 - Gocovri® 137 mg Sample Package, 21 Capsule Bottle Label
-
PRINCIPAL DISPLAY PANELNDC 70482-170-61 - Gocovri® 137 mg Sample Package, 60 Capsule Bottle Label
-
INGREDIENTS AND APPEARANCEProduct Information