Label: GLIADEL- carmustine wafer
- NDC Code(s): 24338-050-08
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLIADEL WAFER safely and effectively. See full prescribing information for GLIADEL WAFER. GLIADEL - ® WAFER (carmustine implant) ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGLIADEL Wafer is indicated for the treatment of patients with: newly-diagnosed high-grade glioma as an adjunct to surgery and radiation, and - recurrent glioblastoma as an adjunct to ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - The recommended dose of GLIADEL Wafer is eight 7.7 mg wafers for a total of 61.6 mg implanted intracranially. The safety and effectiveness of repeat administration have not ...
-
3 DOSAGE FORMS AND STRENGTHSGLIADEL Wafer is an off-white to pale yellow round wafer. Each GLIADEL Wafer contains 7.7 mg of carmustine.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Seizures - Seizures occurred in 37% of patients treated with GLIADEL Wafers for recurrent glioma in Study 2. New or worsening (treatment emergent) seizures occurred in 20% of patients; 54% of ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Seizures - [see - Warnings and Precautions (5.1)] Intracranial Hypertension - [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - GLIADEL Wafer can cause fetal harm when administered to a pregnant woman. There are no available data on GLIADEL use in pregnant women. There have been no animal ...
-
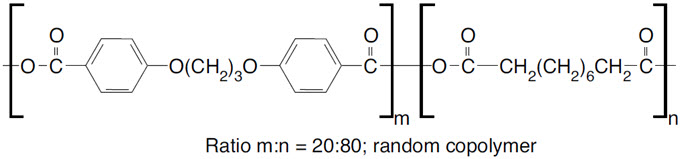
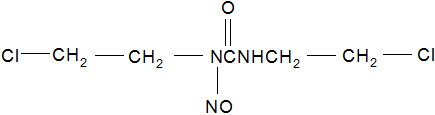
11 DESCRIPTIONGLIADEL Wafer is an implant for intracranial use, containing carmustine, a nitrosourea alkylating agent, and polifeprosan, a biodegradable copolymer used to control the release of carmustine. It ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The activity of GLIADEL Wafer is due to release of cytotoxic concentrations of carmustine, a DNA and RNA alkylating agent, into the tumor resection cavity. On exposure ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, mutagenicity, or impairment of fertility studies have been conducted with GLIADEL Wafer. Carcinogenicity ...
-
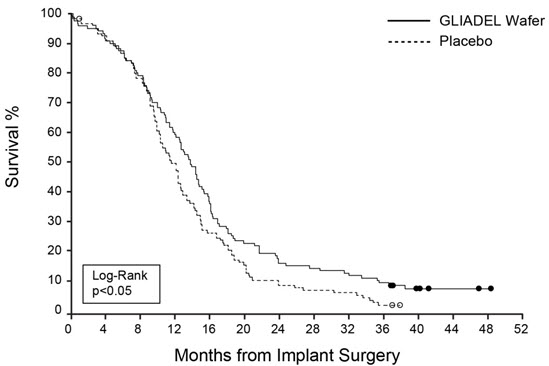
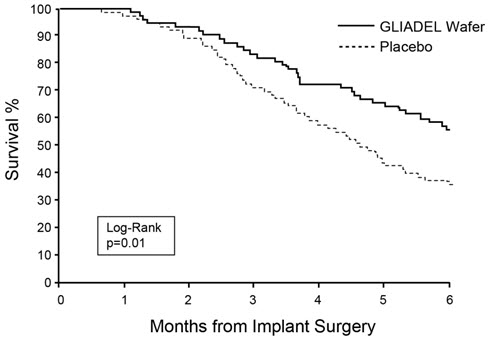
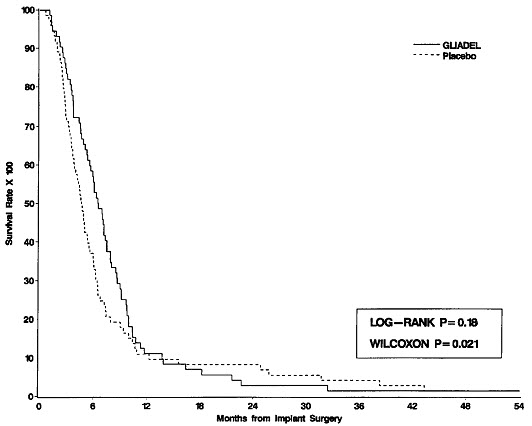
14 CLINICAL STUDIES14.1 Newly-Diagnosed High-Grade Glioma - Study 1 was a multicenter, double-blind, placebo-controlled, clinical trial in adult patients with newly-diagnosed high-grade glioma. A total of 240 ...
-
15 REFERENCES"OSHA Hazardous Drugs". OSHA.http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGLIADEL Wafer is supplied in a single dose treatment box containing eight individually pouched wafers. Each wafer contains 7.7 mg of carmustine and is packaged in two aluminum foil laminate ...
-
17 PATIENT COUNSELING INFORMATIONSeizures - Advise patients to report any new or change in their seizure activity - [see - Warnings and Precautions (5.1)] . Intracranial Hypertension - Advise patients to ...
-
SPL UNCLASSIFIED SECTIONManufactured by - Eisai Inc. Nutley, NJ 07110 - Distributed by - Azurity Pharmaceuticals, Inc. Woburn, MA 01801 - Patent: https://azurity.com/patents - GLIADEL - ®is a ...
-
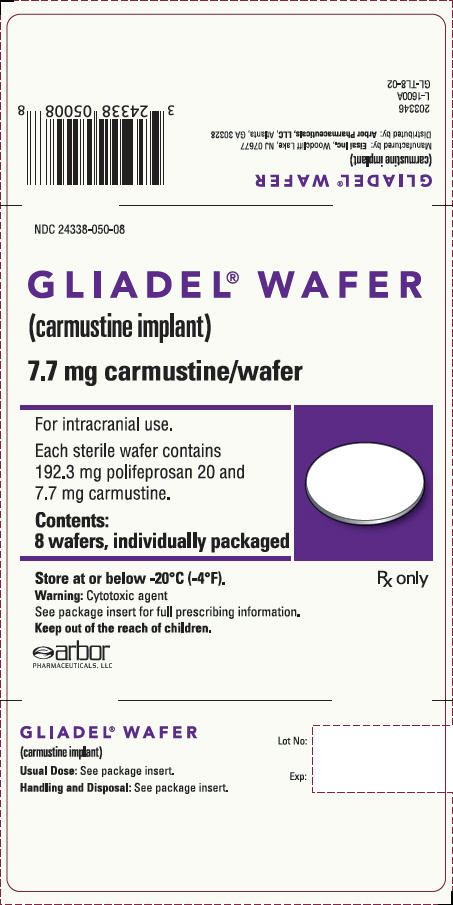
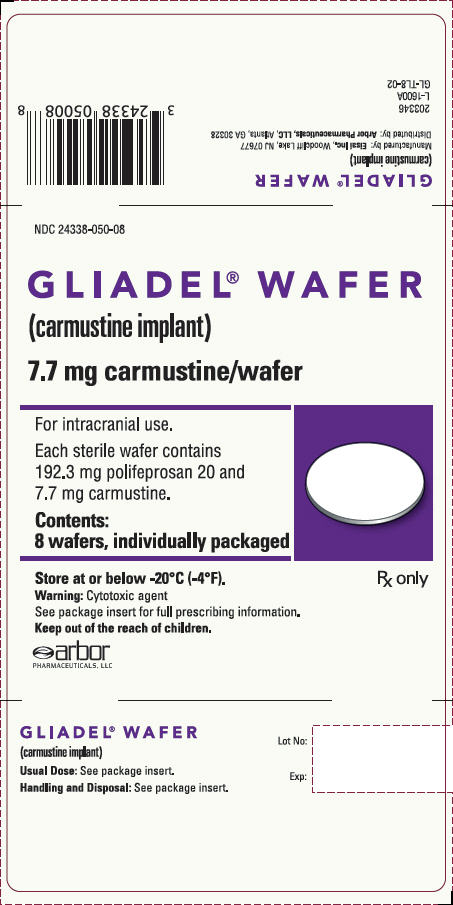
PRINCIPAL DISPLAY PANEL - 8 Wafer BoxNDC 24338-050-08 - GLIADEL - ®WAFER - (carmustine implant) 7.7 mg carmustine/wafer - For intracranial use. Each sterile wafer contains - 192.3 mg polifeprosan 20 and - 7.7 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information