Label: GLEOSTINE- lomustine capsule, gelatin coated
- NDC Code(s): 58181-3040-5, 58181-3041-5, 58181-3042-5
- Packager: NextSource Biotechnology, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLEOSTINE safely and effectively. See full prescribing information for GLEOSTINE. GLEOSTINE - ®(lomustine) capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DELAYED MYELOSUPPRESSION and RISK OF OVERDOSAGE
DELAYED MYELOSUPPRESSION
Gleostine causes myelosuppression including fatal myelosuppression. Myelosuppression is delayed, dose-related, and cumulative; occurring 4 to 6 weeks after drug administration and persisting for 1 to 2 weeks. Thrombocytopenia is generally more severe than leukopenia. Cumulative myelosuppression from Gleostine is manifested by greater severity and longer duration of cytopenias. Monitor blood counts for at least 6 weeks after each dose. Do not give Gleostine more frequently than every 6 weeks [see Warnings and Precautions ( 5.1), Dosage and Administration ( 2.2, 2.3)] .
RISK OF OVERDOSAGE
PRESCRIBE, DISPENSE, AND ADMINISTER ONLY ENOUGH CAPSULES FOR ONE DOSE. Fatal toxicity occurs with overdosage of Gleostine. Both physician and pharmacist should emphasize to the patient that only one dose of Gleostine is taken every 6 weeks [see Dosage and Administration ( 2.1), Warnings and Precautions ( 5.2), Overdosage ( 10)] .
Close -
1 INDICATIONS AND USAGE1.1 Brain Tumors - Gleostine is indicated for the treatment of patients with primary and metastatic brain tumors following appropriate surgical and/or radiotherapeutic procedures. 1.2 ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Prescribing and Dispensing Information - PRESCRIBE ONLY ONE DOSE FOR EACH TREATMENT CYCLE. DO NOT DISPENSE ENTIRE CONTAINER.Dispense only a sufficient number of capsules for one ...
-
3 DOSAGE FORMS AND STRENGTHSGleostine capsules are available in three strengths, distinguishable by the color of the capsules: 100 mg capsules (green/green) 40 mg capsules (white/green) 10 mg capsules (white/white)
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Delayed Myelosuppression - Gleostine causes myelosuppression that can result in fatal infections and bleeding. Myelosuppression from Gleostine is delayed, dose-related, and cumulative. It ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the labeling: Delayed myelosuppression - [see Warnings and Precautions ( 5.1) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal data and its mechanism of action, Gleostine can cause fetal harm when administered to a pregnant woman - [see Clinical Pharmacology ...
-
10 OVERDOSAGEOverdosage with Gleostine has occurred, including fatal cases - [see Dosage and Administration ( 2.1), Warnings and Precautions ( 5.2)]. Overdosage causes severe ...
-
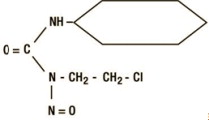
11 DESCRIPTIONGleostine (lomustine) is an alkylating drug for oral administration. The chemical name for lomustine is 1-(2-chloro-ethyl)-3-cyclohexyl-1-nitrosourea and the molecular formula is C - 9H ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lomustine alkylates DNA and RNA. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Lomustine is carcinogenic in rats and mice, producing a marked increase in tumor incidence in doses lower than those employed ...
-
15 REFERENCESOSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Gleostine is available in three strengths, distinguishable by the color of the capsules, in individual bottles of 5 capsules each: Strength - Capsule ...
-
17 PATIENT COUNSELING INFORMATIONMyelosuppression - Advise patients that periodic assessment of their blood counts are required. Advise patients to contact their healthcare provider for new onset of bleeding or fever or ...
-
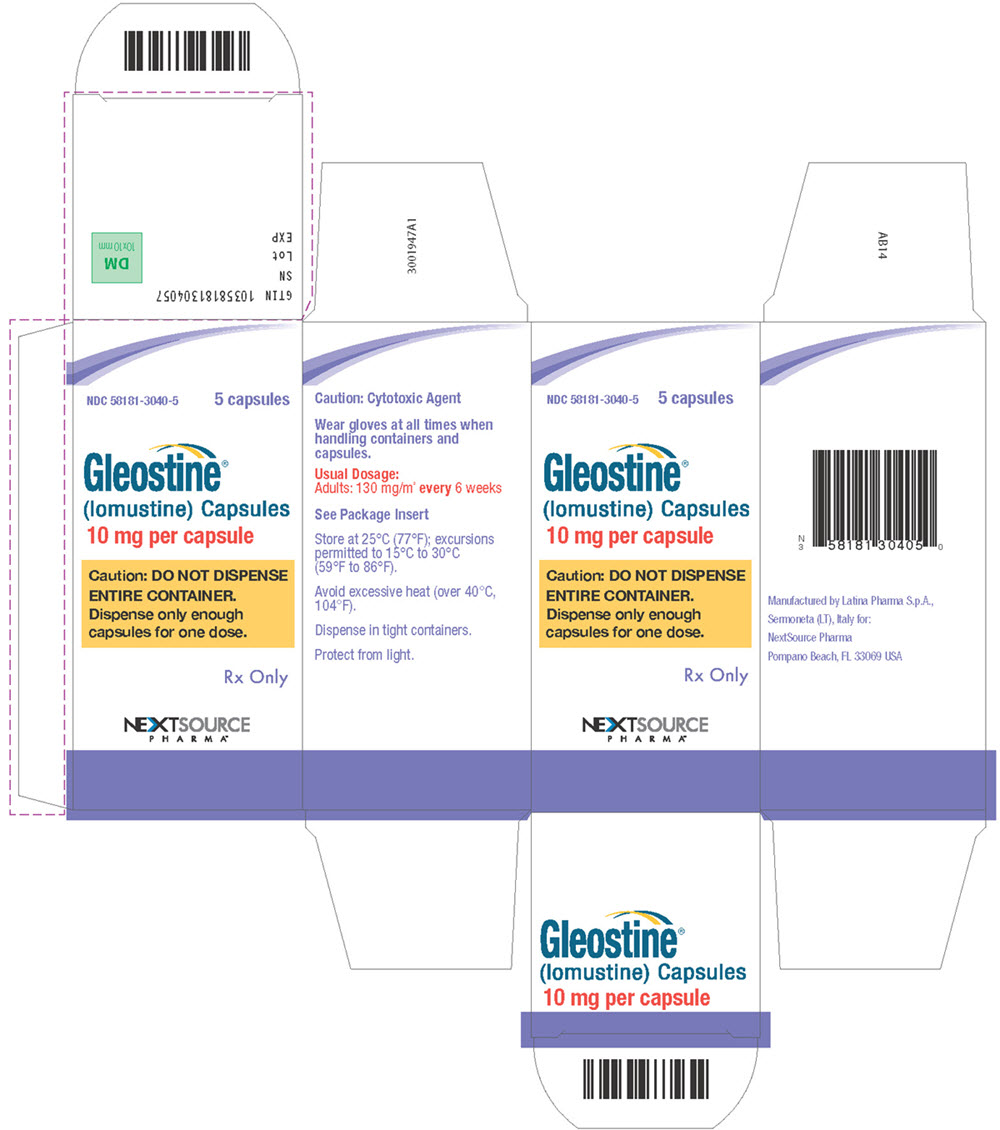
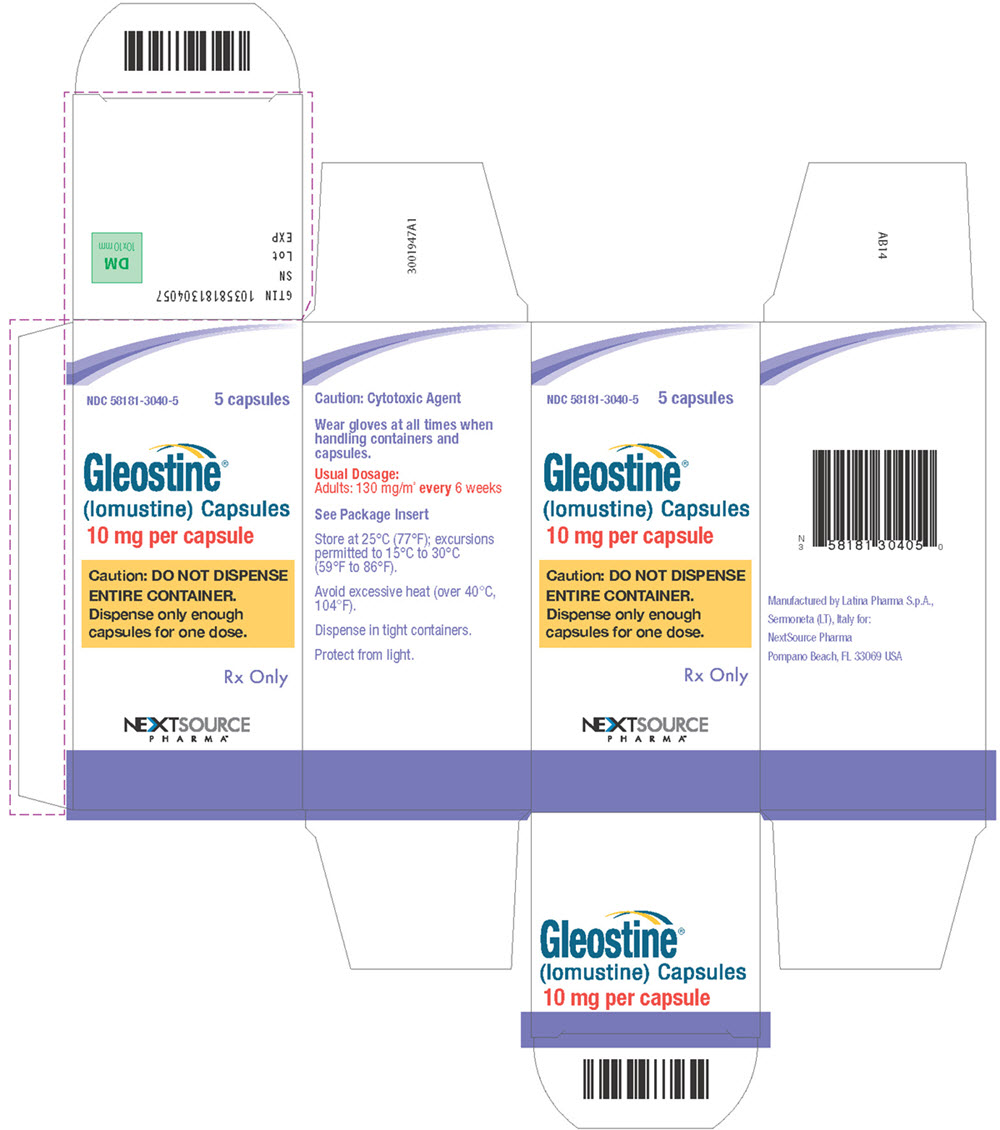
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - NDC58181-3040-5 5 capsules - Gleostine - ® (lomustine) Capsules - 10 mg per capsule - Caution: DO NOT DISPENSE - ENTIRE CONTAINER ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - NDC58181-3041-5 5 capsules - Gleostine - ® (lomustine) Capsules - 40 mg per capsule - Caution: DO NOT DISPENSE - ENTIRE CONTAINER ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - NDC58181-3042-5 5 capsules - Gleostine - ® (lomustine) Capsules - 100 mg per capsule - Caution: DO NOT DISPENSE - ENTIRE CONTAINER ...
-
INGREDIENTS AND APPEARANCEProduct Information