Label: GIAPREZA- angiotensin ii injection
- NDC Code(s): 68547-005-01, 68547-501-02
- Packager: La Jolla Pharmaceutical Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GIAPREZA safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA® (angiotensin II) Injection for ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEGIAPREZA increases blood pressure in adults with septic or other distributive shock [see Clinical Studies (14)].
-
2. DOSAGE AND ADMINISTRATION2.1. Preparation - Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. GIAPREZA must ...
-
3. DOSAGE FORMS AND STRENGTHSInjection: 0.5 mg/mL angiotensin II and 2.5 mg/mL angiotensin II in a vial. GIAPREZA is a clear, aqueous solution.
-
4. CONTRAINDICATIONSNone.
-
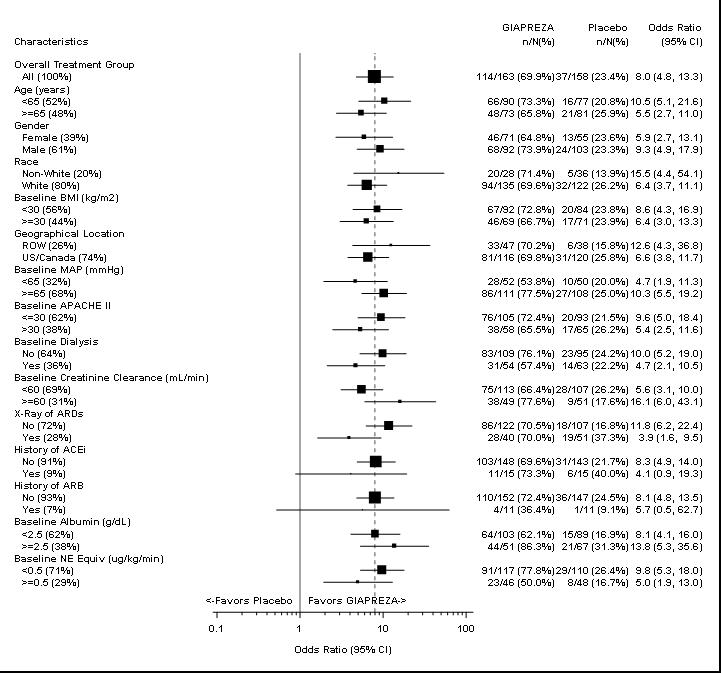
5. WARNINGS AND PRECAUTIONS5.1 Risk for Thrombosis - The safety of GIAPREZA was evaluated in 321 adults with septic or other distributive shock in a randomized, double-blind, placebo-controlled study, ATHOS-3. There was a ...
-
6. ADVERSE REACTIONS6.1. Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7. DRUG INTERACTIONS7.1. Angiotensin Converting Enzyme (ACE) Inhibitors - Concomitant use of angiotensin converting enzyme (ACE) inhibitors may increase the response to GIAPREZA. 7.2. Angiotensin II Receptor ...
-
8. USE IN SPECIFIC POPULATIONS8.1. Pregnancy - Risk Summary - The published data on angiotensin II use in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. Animal ...
-
10. OVERDOSAGEOverdose of GIAPREZA would be expected to result in hypertension, necessitating close monitoring and supportive care. Effects are expected to be brief because the half-life of angiotensin II is ...
-
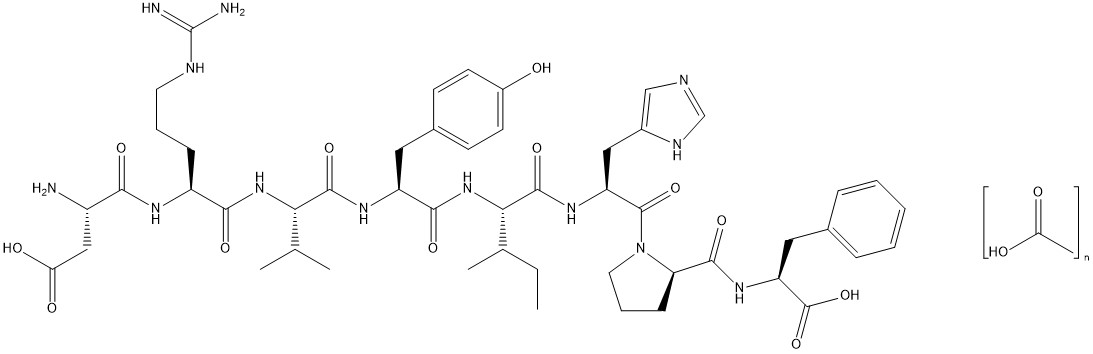
11. DESCRIPTIONAngiotensin II is a naturally occurring peptide hormone of the renin-angiotensin-aldosterone system (RAAS) that causes vasoconstriction and an increase in blood pressure. GIAPREZA is a sterile ...
-
12. CLINICAL PHARMACOLOGY12.1. Mechanism of Action - Angiotensin II raises blood pressure by vasoconstriction and increased aldosterone release. Direct action of angiotensin II on the vessel wall is mediated by binding ...
-
13. NONCLINICAL TOXICOLOGY13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - No genetic toxicity studies have been conducted with GIAPREZA. No carcinogenicity or fertility studies with GIAPREZA have been ...
-
14. CLINICAL STUDIES14.1. ATHOS-3 - The Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial was a double-blind study in which 321 adults with septic or other distributive shock who remained ...
-
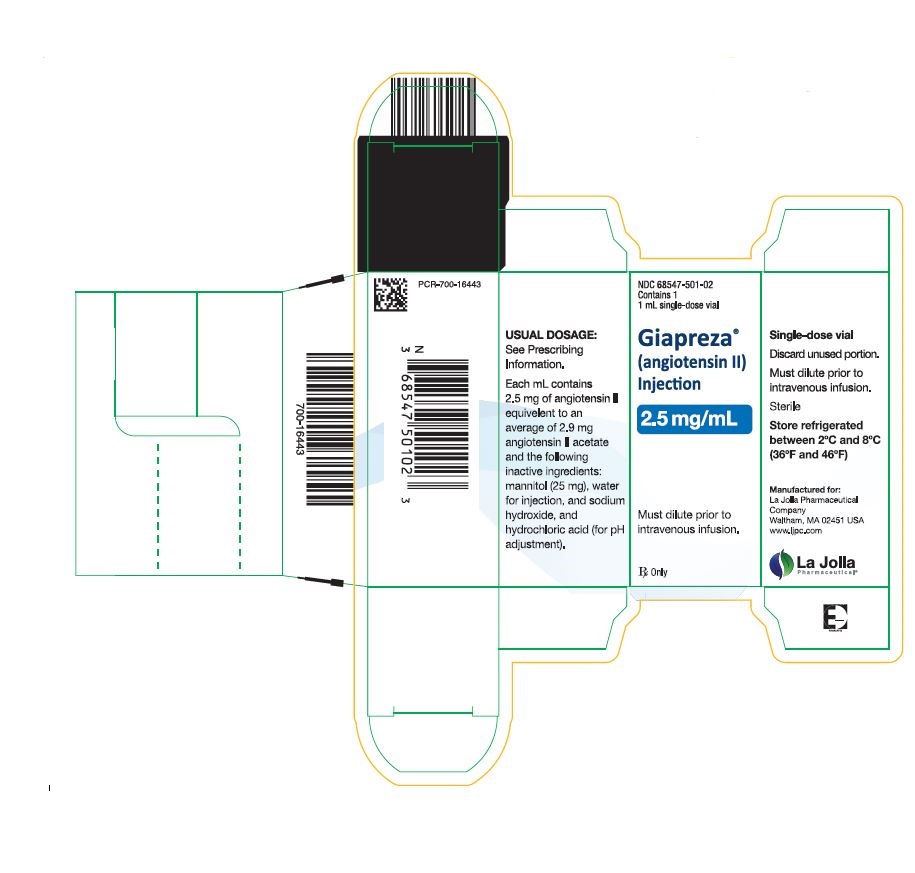
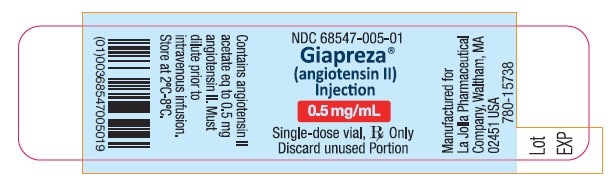
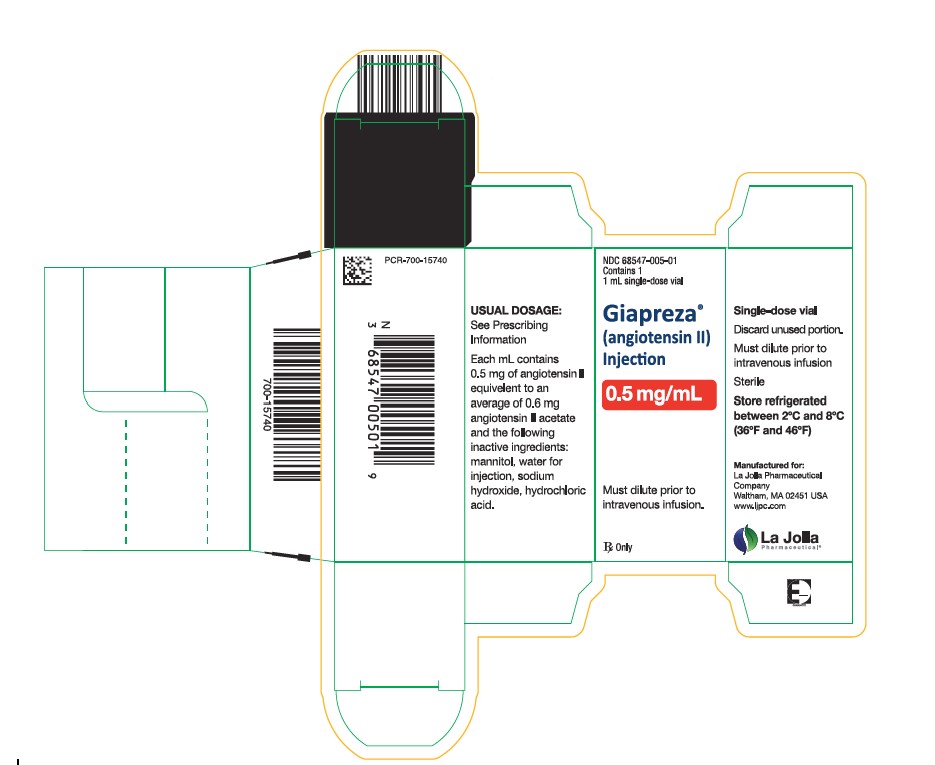
16. HOW SUPPLIED/STORAGE AND HANDLING16.1. How Supplied - GIAPREZA (angiotensin II) Injection is a clear, aqueous solution for administration by intravenous infusion supplied as a single-dose vial: 2.5 mg/mL vial: NDC 68547-501-02 ...
-
SPL UNCLASSIFIED SECTIONManufactured for: La Jolla Pharmaceutical Company - Waltham, MA 02451 USA - GIAPREZA® is a registered trademark of La Jolla Pharmaceutical Company. ©2021, La Jolla Pharmaceutical Company. All rights ...
-
Package Label – 2.5 mg/mL Single-Dose Vial Label

-
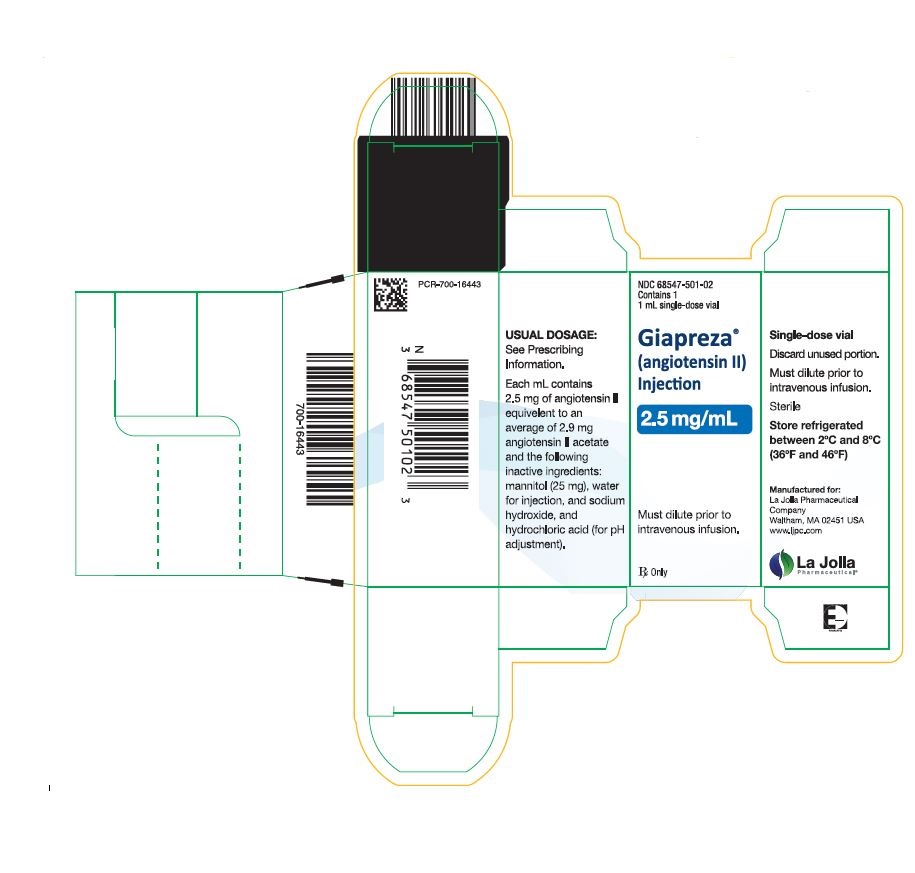
Package Label – 2.5 mg/mL Single Vial Carton Label
-
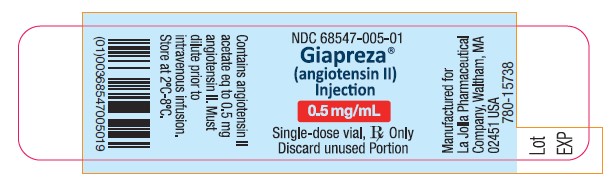
Package Label - 0.5 mg/mL Single-Dose Vial Label
-
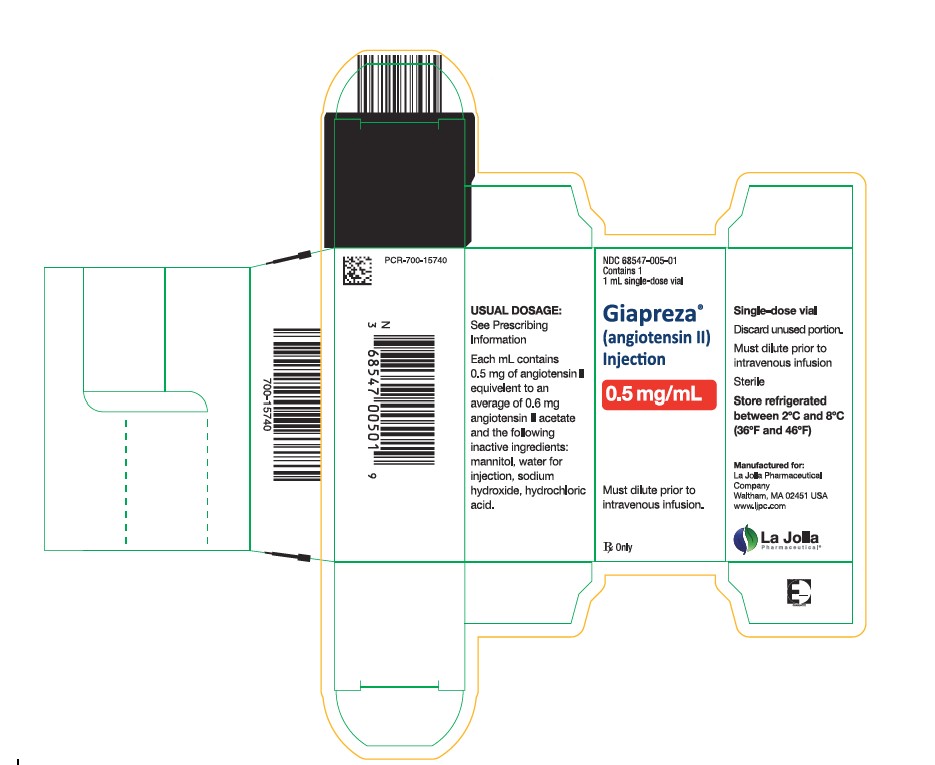
Package Label - 0.5 mg/mL Single Vial Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information