Label: GALZIN- zinc acetate capsule
- NDC Code(s): 57844-208-52, 57844-215-52
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
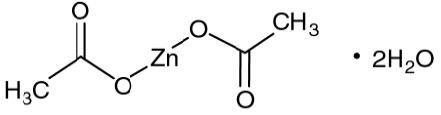
DESCRIPTIONZinc acetate as the dihydrate is a salt of zinc used to inhibit the absorption of copper in patients with Wilson's disease. Its structural formula is: C4H6O4Zn•2H2O M.W. 219.51. Zinc acetate ...
-
CLINICAL PHARMACOLOGYIntroduction - Wilson's disease (hepatolenticular degeneration) is an autosomal recessive metabolic defect in hepatic excretion of copper in the bile, resulting in accumulation of excess copper ...
-
CLINICAL TRIALSIn the single center United States trial, 60 patients with Wilson’s disease (31 male, 29 female) who had adequate detoxification of copper after initial chelation therapy were entered into a ...
-
INDICATIONS AND USAGEZinc acetate therapy is indicated for maintenance treatment of patients with Wilson’s disease who have been initially treated with a chelating agent (See PRECAUTIONS: Monitoring Patients).
-
CONTRAINDICATIONSZinc Acetate Capsules are contraindicated in patients with known hypersensitivity to any of the components of the formulation.
-
WARNINGSCopper Deficiency - Several post-marketing cases reported that zinc acetate taken over extended periods of time (i.e., months to years) may result in decreased enteral copper absorption and ...
-
PRECAUTIONSGeneral - Zinc acetate is not recommended for the initial therapy of symptomatic patients because of the delay required for zinc-induced increase in enterocytic metallothionein and blockade of ...
-
ADVERSE REACTIONSThe following adverse reactions associated with the use of zinc acetate were identified from postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain ...
-
OVERDOSAGEAcute oral overdosage with inorganic salts of zinc in humans is reported rarely. In the event of overdosage, the unabsorbed zinc salt should be removed from the stomach by lavage as quickly as ...
-
DOSAGE AND ADMINISTRATIONThe recommended adult dose is 50 mg as zinc three times daily (See CLINICAL TRIALS). Since 25 mg t.i.d. is also an effective dose in children 10 years of age or older or in women who are pregnant ...
-
HOW SUPPLIEDGALZIN®, Zinc Acetate Capsules (25 mg zinc content) are #1 capsules with aqua blue opaque cap and body, imprinted "93-215." Packaged in bottles of 250 (NDC 57844-215-52). GALZIN® Zinc Acetate ...
-
Package/Label Display PanelNDC 57844-215-52 - GALZIN® 25 mg (zinc acetate) capsules - Rx only - Each aqua blue capsule contains: Zinc Acetate equivalent to 25 mg zinc. 250 CAPSULES - TEVA
-
Package/Label Display PanelNDC 57844-208-52 - GALZIN® 50 mg (zinc acetate) capsules - Rx only - Each orange capsule contains: Zinc Acetate equivalent to 50 mg zinc. 250 CAPSULES - TEVA
-
INGREDIENTS AND APPEARANCEProduct Information