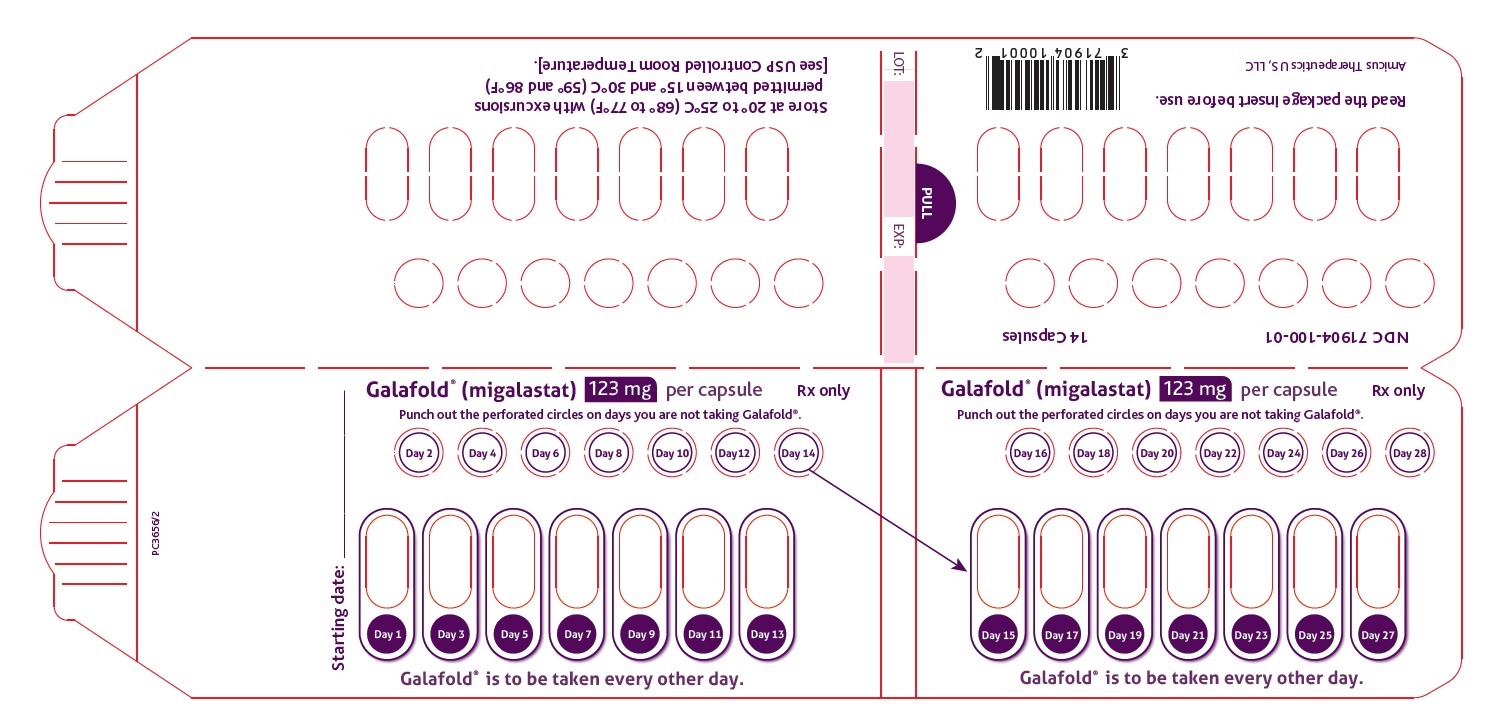
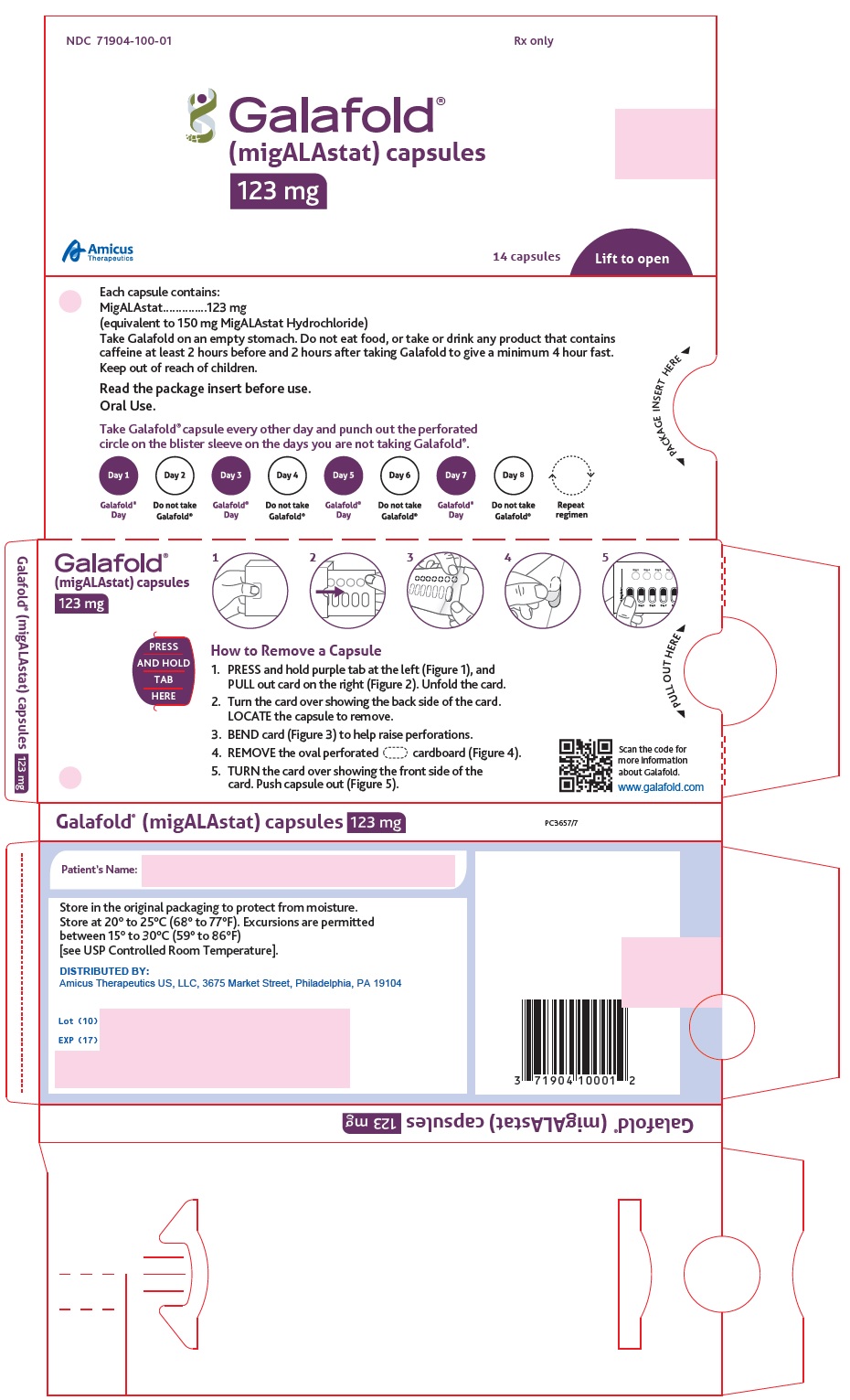
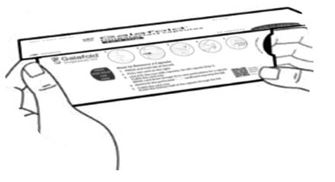
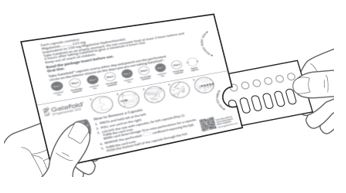
12.1 Mechanism of Action
- Migalastat is a pharmacological chaperone that reversibly binds to the active site of the alpha-galactosidase A (alpha-Gal A) protein (encoded by the galactosidase ...
12.1 Mechanism of Action
Migalastat is a pharmacological chaperone that reversibly binds to the active site of the alpha-galactosidase A (alpha-Gal A) protein (encoded by the galactosidase alpha gene, GLA), which is deficient in Fabry disease. This binding stabilizes alpha-Gal A allowing its trafficking from the endoplasmic reticulum into the lysosome where it exerts its action. In the lysosome, at a lower pH and at a higher concentration of relevant substrates, migalastat dissociates from alpha-Gal A allowing it to break down the glycosphingolipids globotriaosylceramide (GL-3) and globotriaosylsphingosine (lyso-Gb3). Certain GLA variants (mutations) causing Fabry disease result in the production of abnormally folded and less stable forms of the alpha-Gal A protein which, however, retain enzymatic activity. Those GLA variants, referred to as amenable variants, produce alpha-Gal A proteins that may be stabilized by migalastat thereby restoring their trafficking to lysosomes and their intralysosomal activity.
In Vitro Amenability Assay
In an in vitro assay (HEK-293 assay), Human Embryonic Kidney (HEK-293) cell lines were transfected with specific GLA variants which produced variant alpha-Gal A proteins. In the transfected cells, amenability of the GLA variants was assessed after a 5-day incubation with 10 micromol/L migalastat. A GLA variant was categorized as amenable if the resultant variant alpha-Gal A activity (measured in the cell lysates) met two criteria: 1) it showed a relative increase of at least 20% compared to the pre-treatment alpha-Gal A activity, and 2) it showed an absolute increase of at least 3% of the wild-type (normal) alpha-Gal A activity.
The in vitro assay did not evaluate trafficking of the variant alpha-Gal A proteins into the lysosome or the dissociation of migalastat from the variant alpha-Gal A proteins within the lysosome. Also, the in vitro assay did not test whether a GLA variant causes Fabry disease or not.
The GLA variants that are amenable to treatment with GALAFOLD, either based on the in vitro assay data or on the concept that synonymous nucleotide changes leading to the same variant alpha-Gal A protein as a confirmed amenable GLA variant are amenable without additional testing, are shown in Table 2. In patients with multiple identified variants, the amenability assessment of each independent variant may not reflect the overall amenability classification of the combination of variants. The specific variant combination must be present within Table 2 (eg, c.[164A>T; 170A>T]) and on a single chromosome (males and females) to be considered amenable to treatment with GALAFOLD. When multiple variants are identified in a female, it is recommended to consult a clinical genetics professional to determine whether the variants are on a single GLA allele.
Inclusion of GLA variants in this table does not reflect interpretation of their clinical significance in Fabry disease. Whether a certain amenable GLA variant in a patient with Fabry disease is disease-causing or not should be determined by the prescribing physician (in consultation with a clinical genetics professional, if needed) prior to treatment initiation. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease).
If a GLA variant does not appear in Table 2, it is either non-amenable (if tested) or has not been tested for in vitro amenability. For further information, please contact Amicus Medical Information at 1-877-4AMICUS or medinfousa@amicusrx.com.
12.2 Pharmacodynamics
In Study 1, 31 of 50 patients with amenable GLA variants (18 on GALAFOLD, 13 on placebo) had lyso‑Gb3 assessments available after 6 months of treatment. The median change from baseline to month 6 in plasma lyso‑Gb3 (nmol/L) was -2.37 (range -69.7, 1.8) in patients on GALAFOLD and 0.53 (range -21.5, 16.3) in patients on placebo. In the open‑label treatment phase of Study 1, the 13 patients who were initially on placebo for 6 months and who switched to GALAFOLD for another 6 months had a median change in lyso‑Gb3 (nmol/L) of -2.72 (range -61.1, -0.3). The 18 patients who were treated with GALAFOLD for 6 months and then continued GALAFOLD in the open‑label treatment phase of Study 1 for an additional 6 months had no further changes in plasma lyso‑Gb3.
In Study 2, 46 of 56 patients with amenable GLA variants (31 on GALAFOLD, 15 on enzyme replacement therapy (ERT)) had lyso‑Gb3 assessments available after 18 months of treatment. The median change from baseline to month 18 in plasma lyso‑Gb3 (nmol/L) was 0.53 (range -2.27, 28.3) in patients on GALAFOLD and -0.03 (range -11.9, 2.57) in patients on ERT.
Cardiac Electrophysiology
At a dose approximately 8 times the recommended dose, GALAFOLD did not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Following a single GALAFOLD oral dose of 123 mg, the absolute bioavailability (AUC) of migalastat was approximately 75% and the time to peak plasma concentration (tmax) was approximately 3 hours. Plasma migalastat exposure (AUC0‑∞ and Cmax) demonstrated dose‑proportional increases at oral doses from 75 mg to 1250 mg (doses from 0.5 to 8.3‑fold of the approved recommended dosage). Migalastat does not accumulate following administration of 123 mg GALAFOLD every other day.
Effect of Food: Administration of GALAFOLD one hour before a high‑fat (850 calories; 56% from fat) or light meal (507 calories; 30% from fat), or one hour after a light meal, reduced the mean migalastat AUC0‑∞ by 37% to 42% and Cmax by 15% to 39% compared to the fasting state [see Dosage and Administration (2.2)].
Distribution
The apparent volume of distribution (Vz/F) of migalastat in Fabry patients was approximately 89 L (range: 77 to 133 L) at steady state. There was no detectable plasma protein binding following administration of [14C]‑migalastat in the concentration range between 1 to 100 microM.
Elimination
Metabolism: Based upon in vivo data, migalastat is a substrate for uridine diphosphate glucuronosyltransferase (UDPGT), a minor elimination pathway.
Excretion: In a mass balance study in healthy male subjects, following oral administration of 123 mg [14C]‑migalastat, approximately 77% of the total radiolabeled dose was recovered in urine and 20% of the total radiolabeled dose was recovered in feces with an overall total recovery of 98% within 96 hours post‑dose. In urine, unchanged migalastat accounted for 80% of the radioactivity, which equates to 62% of the administered dose. In feces, unchanged migalastat was the only drug‑related component. In plasma, unchanged migalastat accounted for approximately 77% of the plasma radioactivity and three dehydrogenated O‑glucuronide conjugated metabolites, M1 to M3, together accounted for approximately 13% of the plasma radioactivity, none of which comprised more than 6% of the radiolabeled dose. Approximately 9% of the total radioactivity in plasma was unassigned.
Following a single oral dose of 123 mg GALAFOLD, migalastat is cleared from plasma with a mean half‑life (t½) of approximately 4 hours and apparent clearance of 12.5 L/hr.
Specific Populations
Male and Female Patients: The pharmacokinetic characteristics of migalastat were not significantly different between healthy male and female subjects or patients with Fabry disease.
Racial or Ethnic Groups: Clinical data indicate no ethnic differences in patient populations studied with migalastat.
Patients with Renal Impairment: In a single‑dose study in subjects with varying degrees of renal impairment, exposure to migalastat (AUC) was increased by 1.2-, 1.8-, and 4.3-fold in subjects with mild (eGFR 60 to 90 mL/min/1.73 m2), moderate (eGFR 30 to 59 mL/min/1.73 m2), and severe renal impairment (eGFR less than 30 mL/min/1.73 m2), respectively, while the Cmax remained unchanged with severity of renal impairment [see Use in Specific Populations (8.6)].
Drug Interaction Studies
In Vitro Studies
Migalastat is not a known inhibitor or inducer of cytochrome P450 (CYP450) enzymes, nor is it an inhibitor of BCRP, MDR1, P‑glycoprotein (P‑gp), or BSEP human efflux transporters, or OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2‑K human uptake transporters. Migalastat is not a substrate of P‑gp, BCRP, MDR1 or MATE1, MATE2‑K, OAT1, OAT3, or OCT2. Migalastat showed low affinity for SGLT1, as both a substrate and an inhibitor, and showed no activity for SGLT2.
Clinical Studies: Effects of other Drugs on Migalastat
Co‑administration of 190 mg caffeine reduced the mean migalastat AUC0‑∞ by 55% and Cmax by 60% compared to without caffeine co‑administration. The tmax of migalastat was not affected by co‑administration of caffeine. No clinically significant pharmacokinetic changes were observed for migalastat when co‑administered with sucrose, aspartame or acesulfame potassium [see Dosage and Administration (2.2) and Drug Interactions (7.1)].
Close