Label: FYAVOLV- norethindrone acetate and ethinyl estradiol tablet, film coated
- NDC Code(s): 68180-827-09, 68180-827-13, 68180-827-73, 68180-828-09, view more
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FYAVOLV safely and effectively. See full prescribing information for FYAVOLV. FyavolvTM (norethindrone acetate and ethinyl estradiol ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Estrogen Plus Progestin Therapy - Cardiovascular Disorders and Probable Dementia - The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of pulmonary ...
WARNING: CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA, BREAST CANCER, and ENDOMETRIAL CANCER
CloseEstrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of pulmonary embolism (PE), deep vein thrombosis (DVT), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see Warnings and Precautions (5.1) and Clinical Studies (14.5)].
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.6)].
Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.5, 14.6)].
Breast Cancer
The WHI estrogen plus progestin substudy demonstrated an increased risk of invasive breast cancer [see Warnings and Precautions (5.2) and Clinical Studies (14.5)].
Once daily oral 0.625 mg CE and 2.5 mg MPA were studied in the estrogen plus progestin substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events, dementia and breast cancer to lower CE plus other MPA doses, other routes of administration, or other estrogen plus progestogen products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen plus progestogen therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding [see Warnings and Precautions (5.2)].
Cardiovascular Disorders and Probable Dementia
The WHI estrogen-alone substudy reported increased risks of stroke and DVT in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral CE (0.625 mg)-alone, relative to placebo [see Warnings and Precautions (5.1) and Clinical Studies (14.5)].
The WHIMS estrogen-alone ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5) and Clinical Studies (14.6)].
Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.5, 14.6)].
Only daily oral 0.625 mg CE was studied in the estrogen-alone substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events and dementia to lower CE doses, other routes of administration, or other estrogen-alone products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen-alone therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
1 INDICATIONS AND USAGE1.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause - 1.2 Prevention of Postmenopausal Osteoporosis - Limitation of Use - When prescribing solely for the prevention of ...
-
2 DOSAGE AND ADMINISTRATIONUse estrogen, alone or in combination with a progestogen, at the lowest effective dose and the shortest duration consistent with treatment goals and risks for the individual woman. Re-evaluate ...
-
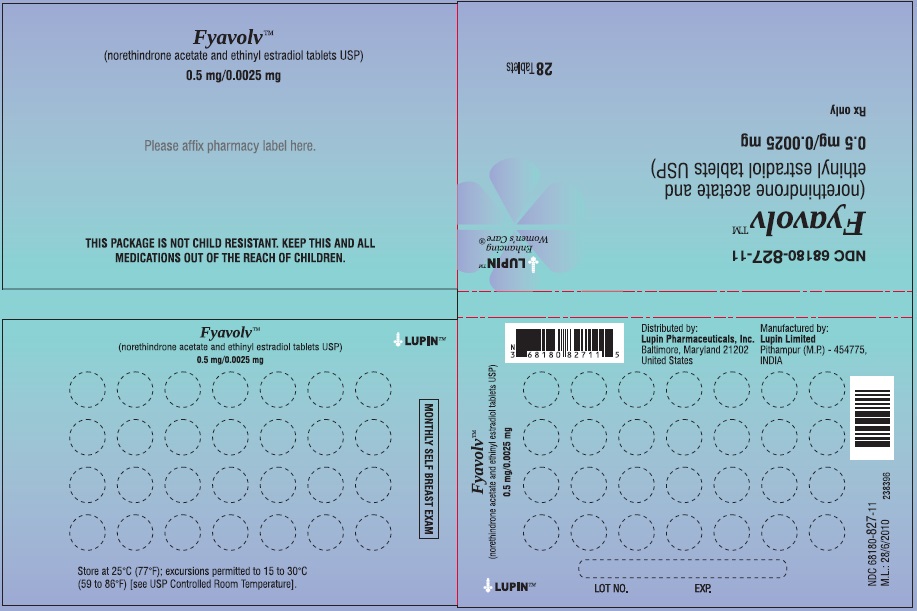
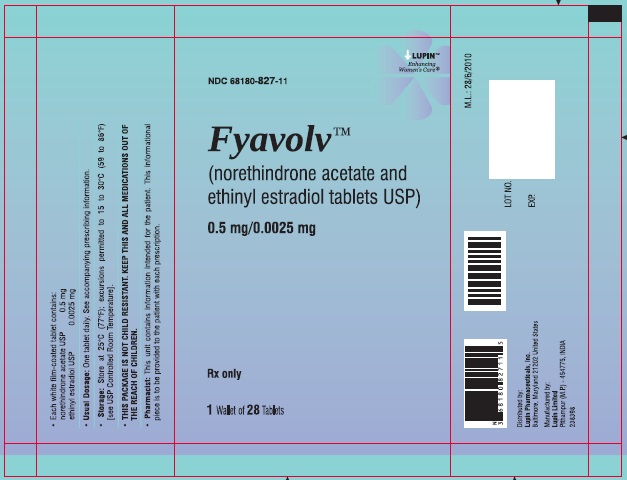
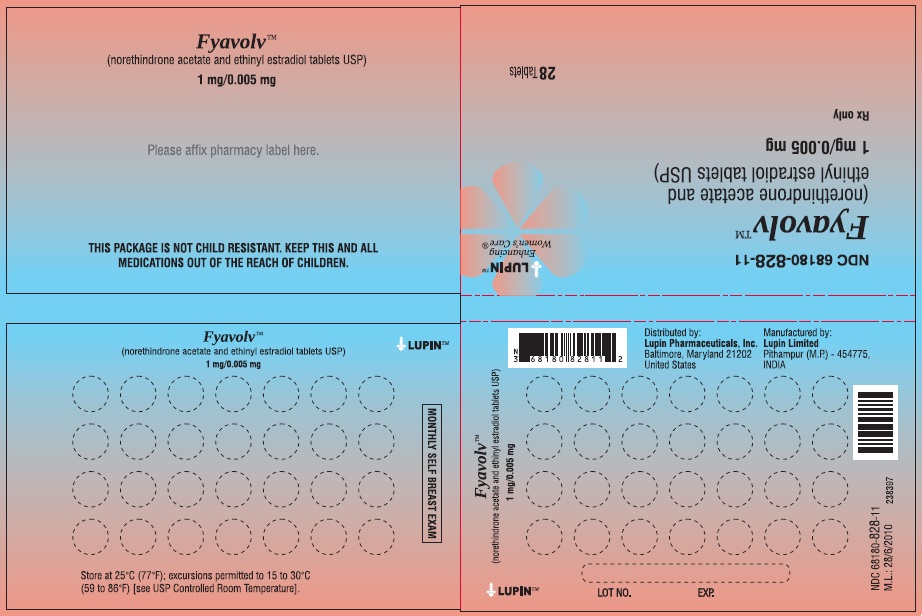
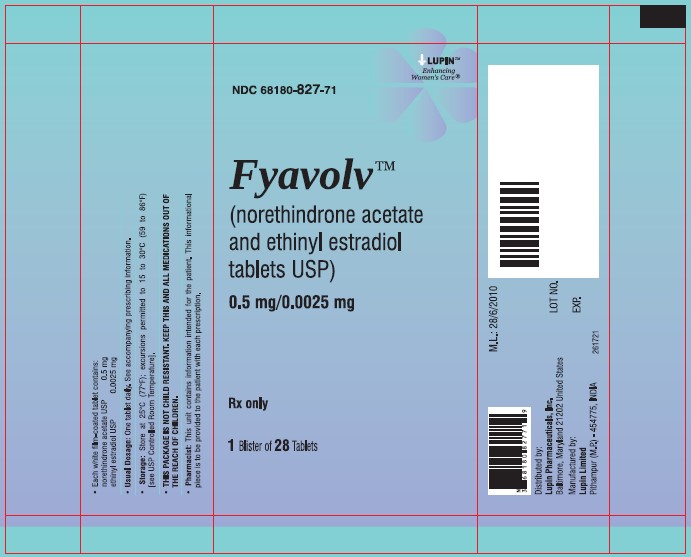
3 DOSAGE FORMS AND STRENGTHSThe following two strengths of Fyavolv tablets are available: Fyavolv tablets, (0.5 mg/0.0025 mg): Each are white to off-white, round film-coated tablet, debossed with "F51" on one side and ...
-
4 CONTRAINDICATIONSFyavolv is contraindicated in women with any of the following conditions: Undiagnosed abnormal genital bleeding [see Warnings and Precautions (5.2)]. Breast cancer or a history of breast ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Disorders - Increased risks of PE, DVT, stroke, and MI are reported with estrogen plus progestin therapy. Increased risks of stroke and DVT are reported with estrogen-alone ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Cardiovascular Disorders [see Boxed Warning, Warnings and Precautions (5.1)]. Malignant Neoplasms [see Boxed ...
-
7 DRUG INTERACTIONSIn vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen and progestin ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Norethindrone acetate and ethinyl estradiol is not indicated for use in pregnancy. There are no data with the use of norethindrone acetate and ethinyl estradiol in ...
-
10 OVERDOSAGEOverdosage of estrogen plus progestogen may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose ...
-
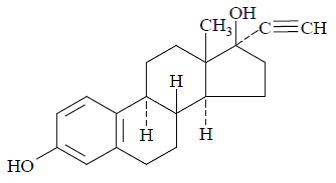
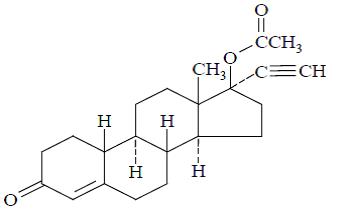
11 DESCRIPTIONFyavolv (norethindrone acetate and ethinyl estradiol tablets USP) is a continuous dosage regimen of a progestin-estrogen combination for oral administration. The following strength of Fyavolv ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas ...
-
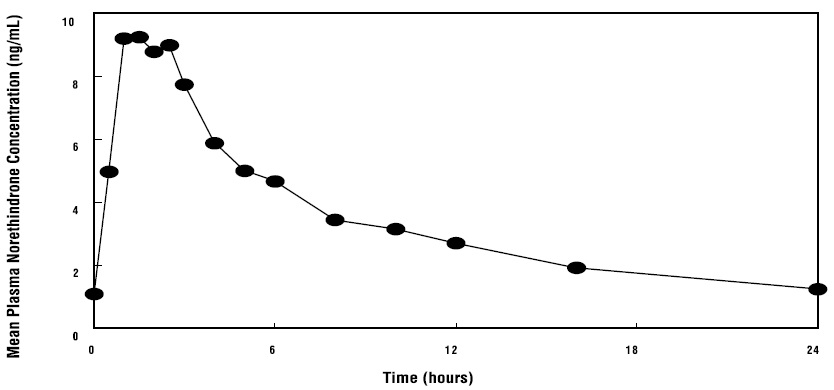
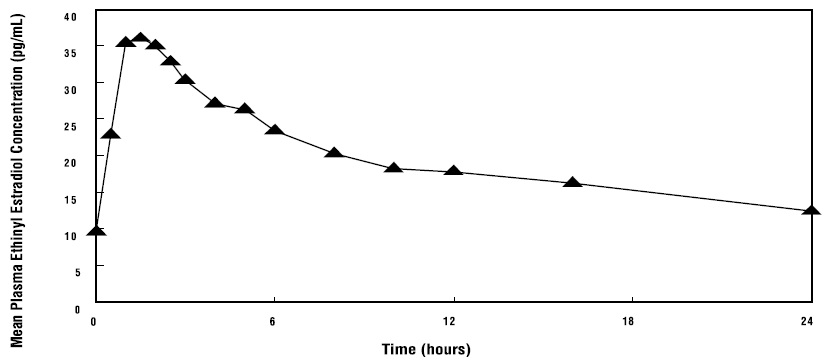
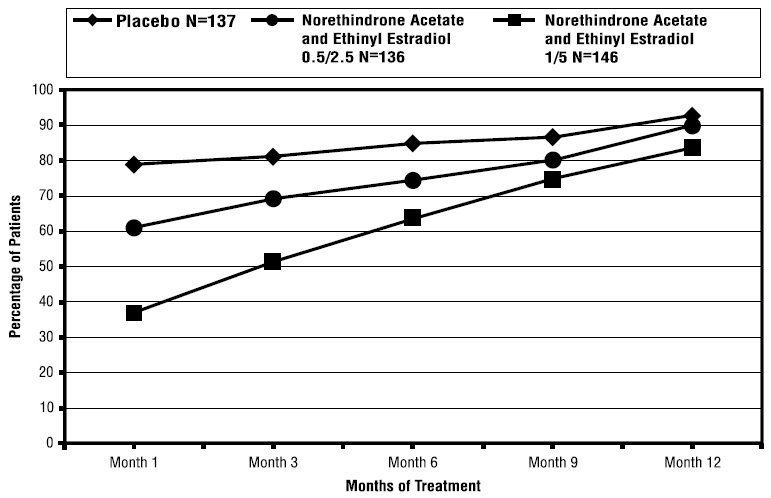
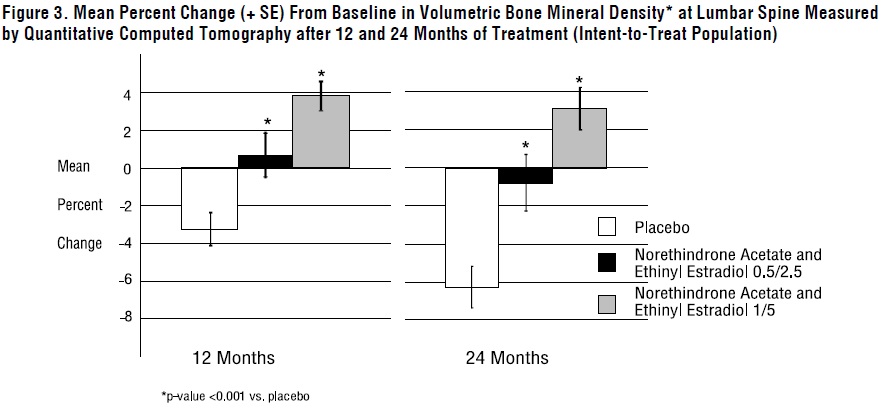
14 CLINICAL STUDIES14.1 Effects on Vasomotor Symptoms in Postmenopausal Women - A 12-week placebo-controlled, multicenter, randomized clinical trial was conducted in 266 symptomatic women who had at least 56 ...
-
15 REFERENCESRossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause.JAMA. 2007;297:1465-1477. Hsia J, et al. Conjugated Equine Estrogens and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Fyavolv 0.5 mg/0.0025 mg are white to off-white, round film-coated tablets, debossed with "F51" on one side and "LU" on the other side, containing 0.5 mg of norethindrone ...
-
17 PATIENT COUNSELING INFORMATIONAdvise women to read the FDA-approved patient labeling (Patient Information) Vaginal Bleeding - Inform postmenopausal women to report any vaginal bleeding to their healthcare provider as ...
-
Patient InformationFyavolv™ (FYE-uh-volv) (norethindrone acetate and ethinyl estradiol tablets USP) 0.5 mg/0.0025 mg and 1 mg/0.005 mg - Read this Patient Information before you start using Fyavolv and each ...
-
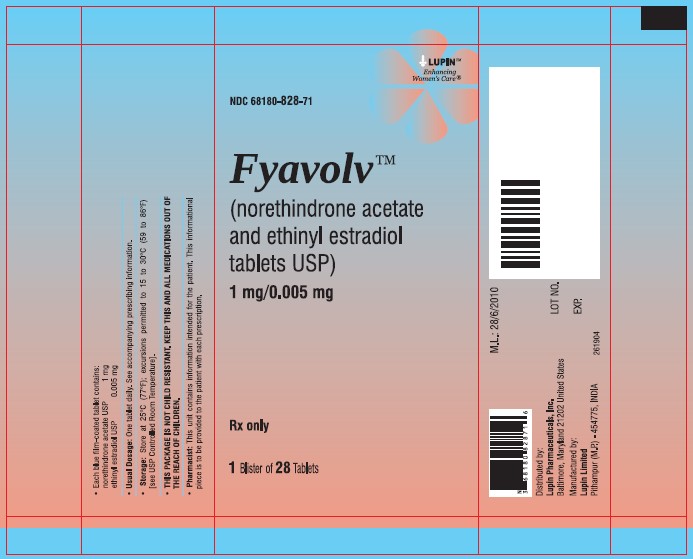
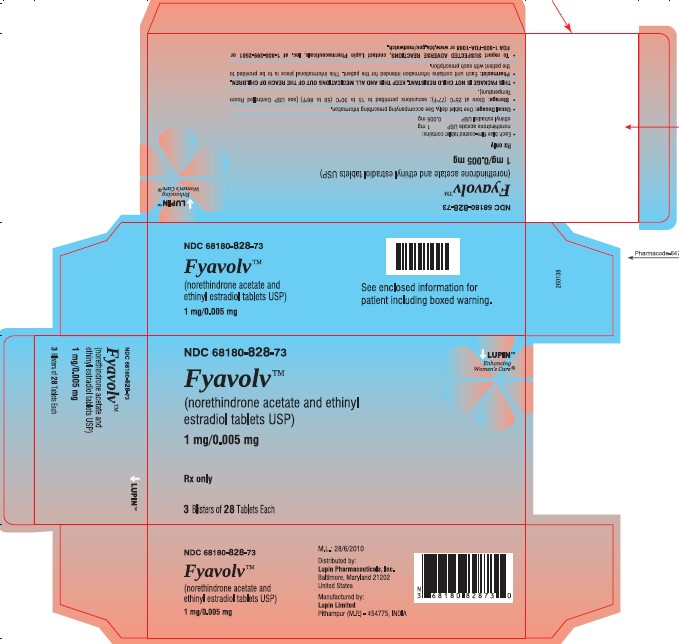
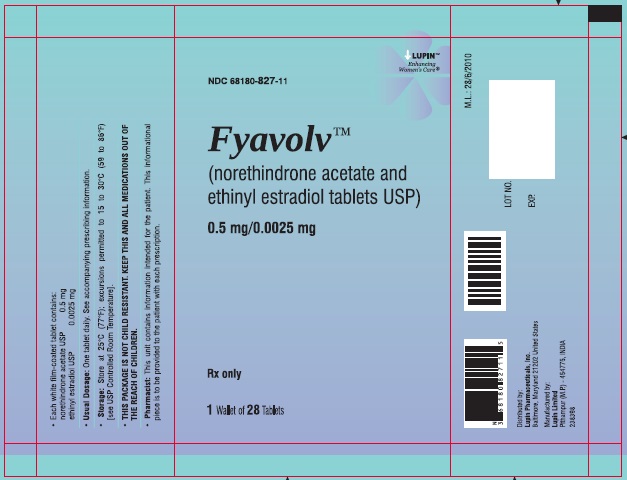
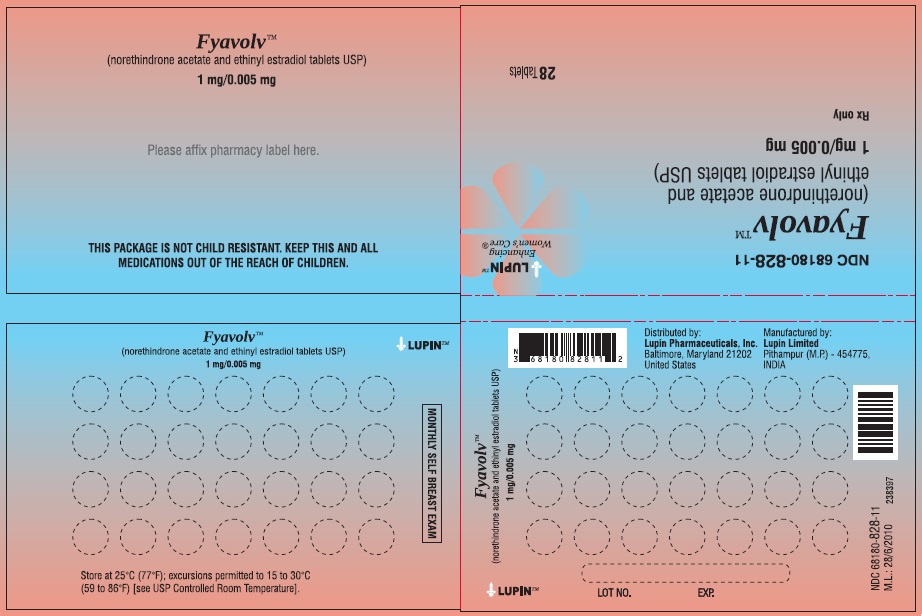
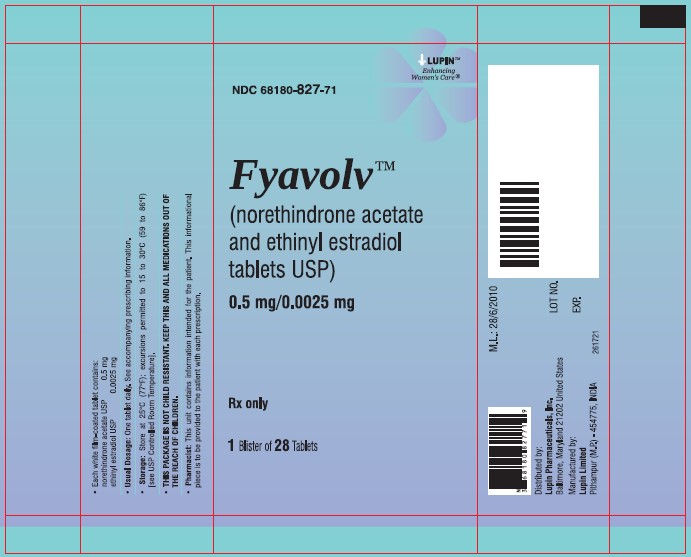
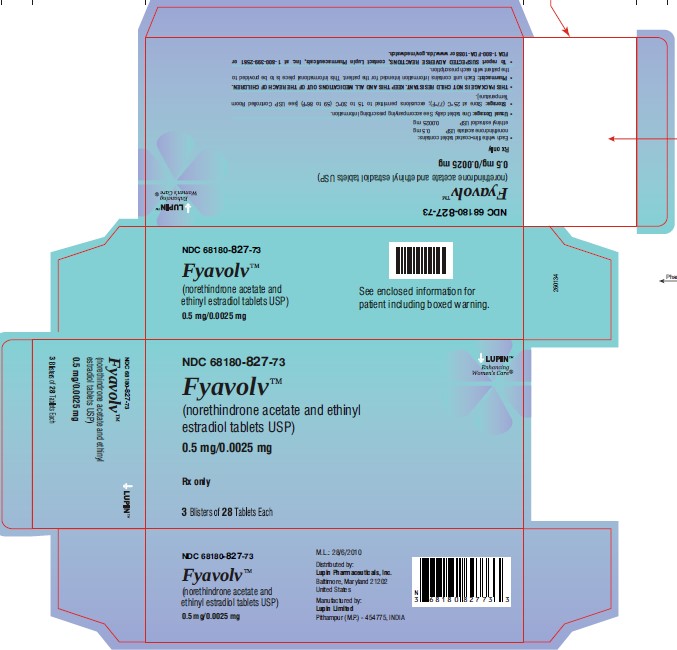
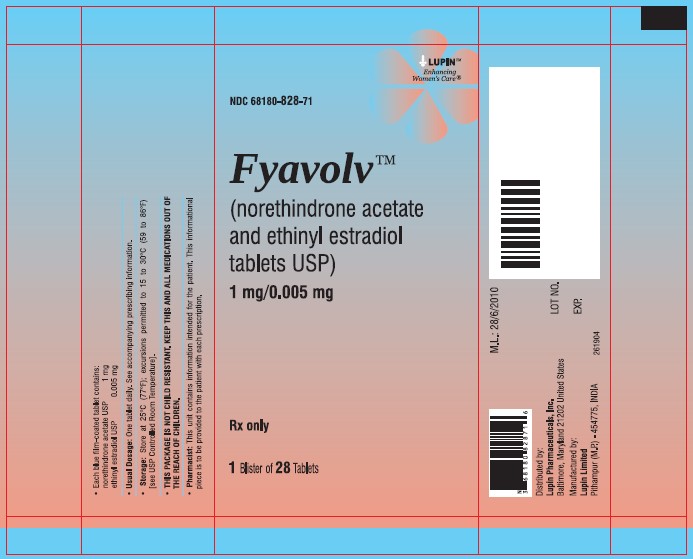
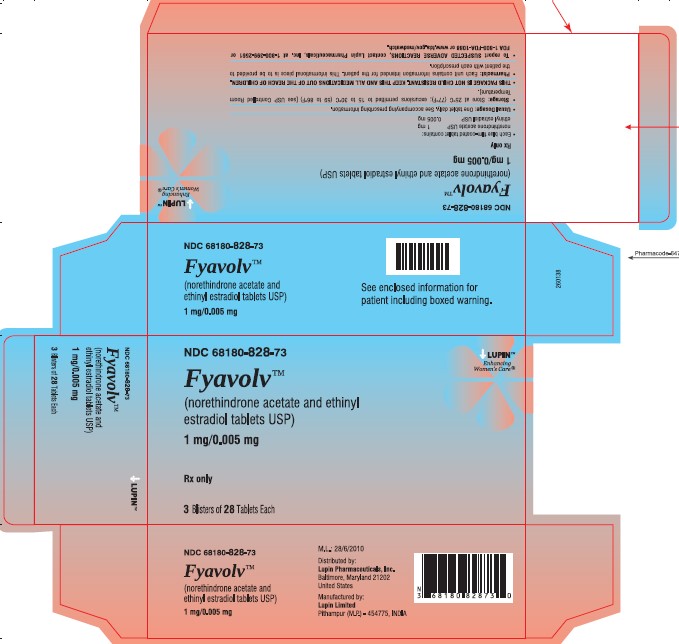
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFyavolv™ (norethindrone and ethinyl estradiol tablets USP) 0.5 mg/0.0025 mg - Rx Only - Wallet and Pouch Label: 28 Tablets - NDC 68180-827-11 - Carton Label: 3 Wallets of 28 Tablets Each - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information