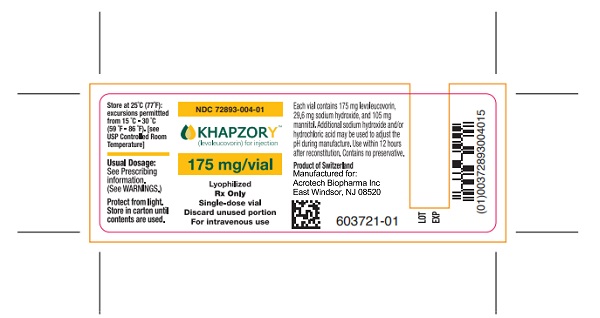
Label: KHAPZORY- levoleucovorin injection, powder, lyophilized, for solution
- NDC Code(s): 72893-004-01
- Packager: Acrotech Biopharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KHAPZORY® safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEKHAPZORY is indicated for: rescue after high-dose methotrexate therapy in adult and pediatric patients with osteosarcoma. diminishing the toxicity associated with overdosage of folic acid ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Use Information - KHAPZORY is indicated for intravenous administration only. Do not administer intrathecally. 2.2 Recommended Dosage for Rescue After High-Dose Methotrexate ...
-
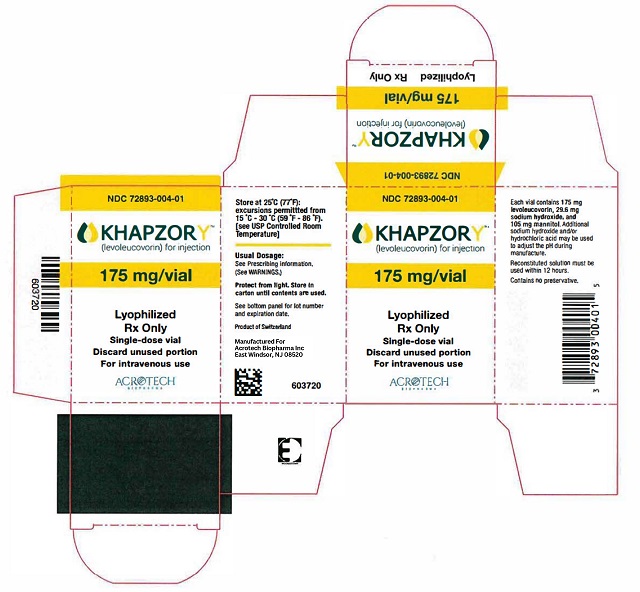
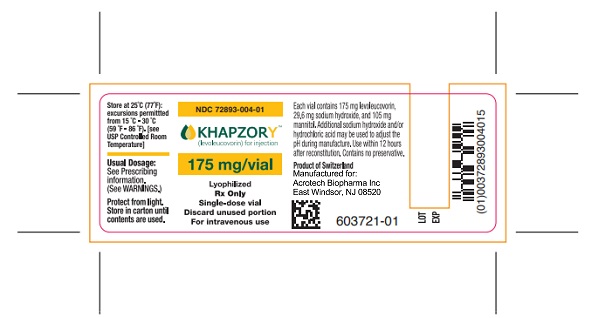
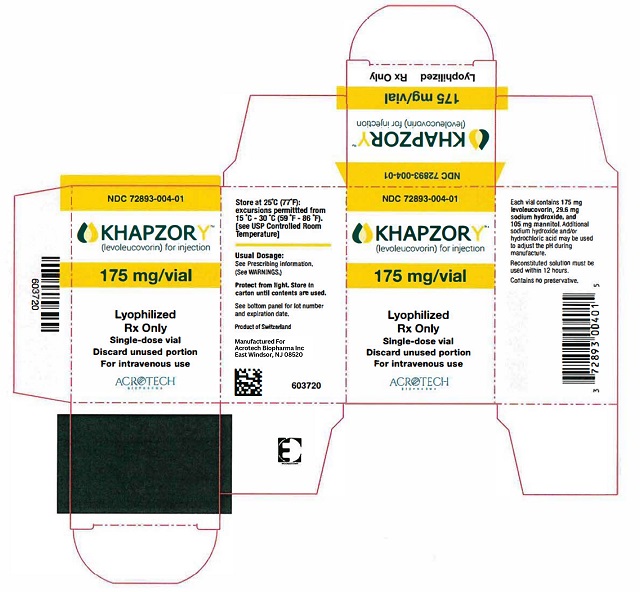
3 DOSAGE FORMS AND STRENGTHSFor Injection: 175 mg of levoleucovorin as a sterile, white to yellowish lyophilized powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSKHAPZORY is contraindicated in patients who have had severe hypersensitivity to leucovorin products, folic acid, or folinic acid [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Increased Gastrointestinal Toxicities with Fluorouracil - Leucovorin products increase the toxicities of fluorouracil [see Drug Interactions (7)]. Gastrointestinal toxicities, including ...
-
6 ADVERSE REACTIONSThe following clinical significant adverse reactions are described elsewhere in the labeling: • Increased gastrointestinal toxicities with fluorouracil [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effects of Leucovorin Products on Other Drugs - Antiepileptic Drugs - Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are limited data with levoleucovorin use in pregnant women. Animal reproduction studies have not been conducted with levoleucovorin. Levoleucovorin is ...
-
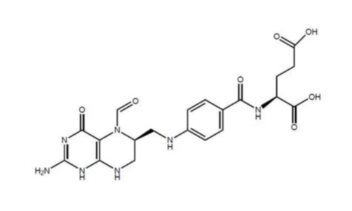
11 DESCRIPTIONLevoleucovorin is a folate analog and the pharmacologically active levo-isomer of d,l-leucovorin. The chemical name is (2S)-2-[[4-[[(6S)-2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6- yl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - High-Dose Methotrexate Therapy - Levoleucovorin is the pharmacologically active isomer of 5-formyl tetrahydrofolic acid. Levoleucovorin does not require reduction by ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to evaluate the potential of levoleucovorin for carcinogenesis, mutagenesis and impairment of ...
-
14 CLINICAL STUDIES14.1 Rescue after High-Dose Methotrexate Therapy in Patients with Osteosarcoma - The efficacy of levoleucovorin rescue following high-dose methotrexate were evaluated in 16 patients aged 6 to 21 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKHAPZORY (levoleucovorin) for injection is a sterile, preservative-free, white to yellowish lyophilized powder in a single-dose vial. It is available as: 175 mg vial –NDC 72893-004-01. Store at ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELCarton Label - NDC 72893-004-01 - KHAPZORY - 175 mg/vial - For Intravenous use only - Single-dose vial - Vial Label - NDC 72893-004-01 - KHAPZORY - 175 mg/vial - For Intravenous use only - Single-dose ...
-
INGREDIENTS AND APPEARANCEProduct Information