Label: FRAGMIN- dalteparin sodium injection
- NDC Code(s): 0069-0195-01, 0069-0195-02, 0069-0196-01, 0069-0196-02, view more
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FRAGMIN safely and effectively. See full prescribing information for FRAGMIN. FRAGMIN® (dalteparin sodium) injection, USP, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SPINAL/EPIDURAL HEMATOMAS
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- •
- Use of indwelling epidural catheters
- •
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants.
- •
- A history of traumatic or repeated epidural or spinal punctures
- •
- A history of spinal deformity or spinal surgery
- •
- Optimal timing between the administration of FRAGMIN and neuraxial procedures is not known
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary.
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.1) and Drug Interactions (7)].
Close -
1 INDICATIONS AND USAGE1.1 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction - FRAGMIN Injection is indicated for the prophylaxis of ischemic complications in unstable angina ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction - In patients with unstable angina or non-Q-wave myocardial infarction ...
-
3 DOSAGE FORMS AND STRENGTHSFRAGMIN (dalteparin sodium) injection is a sterile, aqueous, clear, colorless or straw-colored solution for injection, available in the following dosage forms and strengths: • Injection: 2,500 ...
-
4 CONTRAINDICATIONSFRAGMIN is contraindicated in: • Patients with active major bleeding. • Patients with a history of heparin induced thrombocytopenia or heparin induced thrombocytopenia with thrombosis. • Patients ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Hemorrhage including Spinal/Epidural Hematomas - Spinal or epidural hemorrhage and subsequent hematomas can occur with the associated use of low molecular weight heparins or ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described in more detail in other sections of the prescribing information. • Risk of Hemorrhage including Spinal/Epidural Hematomas ...
-
7 DRUG INTERACTIONSThe use of FRAGMIN in patients receiving oral anticoagulants, platelet inhibitors, and thrombolytic agents may increase the risk of bleeding [see Warnings and Precautions (5)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing reports have not reported a clear association with FRAGMIN and adverse developmental outcomes. There ...
-
10 OVERDOSAGEAn excessive dosage of FRAGMIN Injection may lead to hemorrhagic complications. These may generally be stopped by slow intravenous injection of protamine sulfate (1% solution), at a dose of 1 mg ...
-
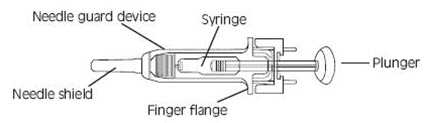
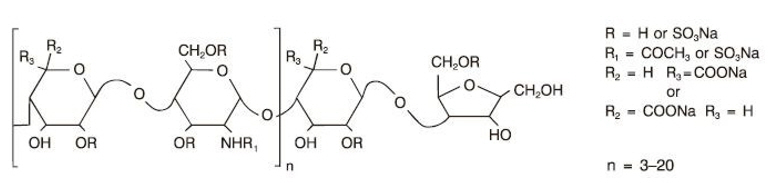
11 DESCRIPTIONFRAGMIN Injection (dalteparin sodium injection) is a sterile, low molecular weight heparin. It is available in single-dose, prefilled syringes preassembled with a needle guard device, and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dalteparin is a low molecular weight heparin with antithrombotic properties. It acts by enhancing the inhibition of Factor Xa and thrombin by antithrombin. In humans ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Dalteparin sodium has not been tested for its carcinogenic potential in long-term animal studies. It was not mutagenic in the in vitro ...
-
14 CLINICAL STUDIES14.1 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction - In a double-blind, randomized, placebo-controlled clinical trial, patients who recently ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFRAGMIN (dalteparin sodium) injection, USP, is a sterile, aqueous, clear, colorless or straw-colored solution available as follows: * Single-dose prefilled syringe, affixed with a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Risk of Hemorrhage including Spinal/Epidural Hematomas - If patients have had neuraxial anesthesia or spinal ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. FRAGMIN is a registered trademark of Pfizer Health AB. LAB-0058-25.0
-
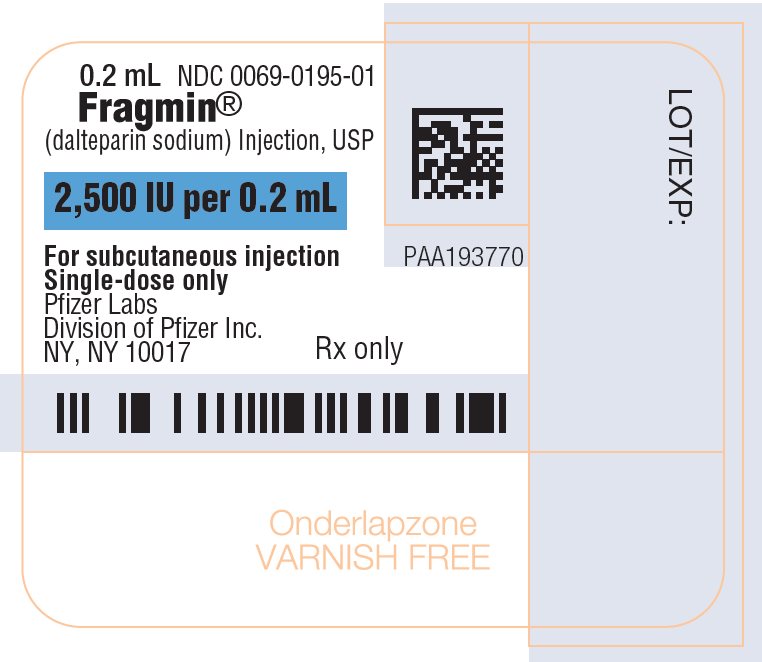
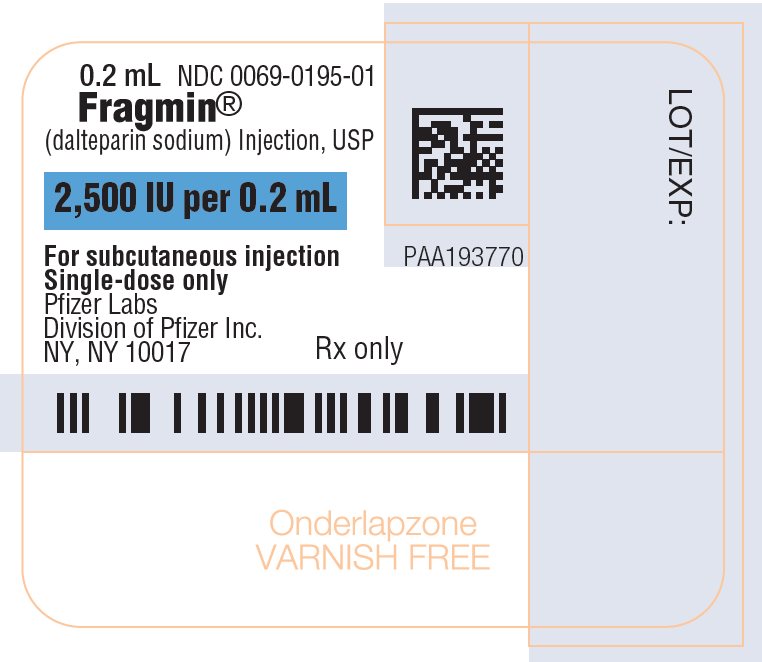
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Label - 01950.2 mL - NDC 0069-0195-01 - Fragmin® (dalteparin sodium) Injection, USP - 2,500 IU per 0.2 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Blister Pack Label - 01950.2 mL Single-dose prefilled syringe - NDC 0069-0195-01 - Fragmin® (dalteparin sodium) Injection, USP - 2,500 IU per 0.2 mL - For subcutaneous injection - Rx only - PAA194311
-
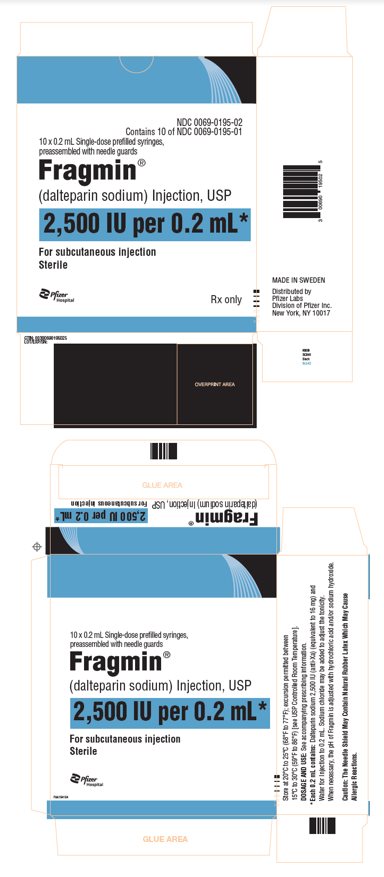
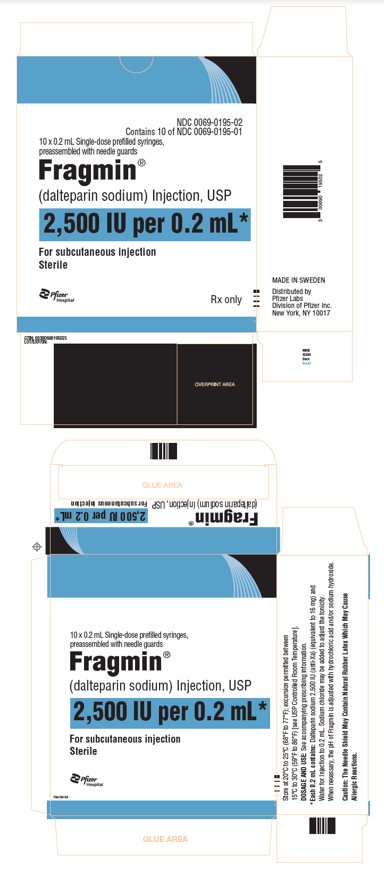
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Carton - 0195NDC 0069-0195-02 - Contains 10 of NDC 0069-0195-01 - 10 x 0.2 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 2,500 IU per 0.2 mL* For ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Label - 01960.2 mL - NDC 0069-0196-01 - Fragmin® (dalteparin sodium) Injection, USP - 5,000 IU per 0.2 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Blister Pack Label - 01960.2 mL Single-dose prefilled syringe - NDC 0069-0196-01 - Fragmin® (dalteparin sodium) Injection, USP - 5,000 IU per 0.2 mL - For subcutaneous injection - Rx only - PAA194306
-
PRINCIPAL DISPLAY PANEL - 0.2 mL Syringe Carton - 0196NDC 0069-0196-02 - Contains 10 of NDC 0069-0196-01 - 10 x 0.2 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 5,000 IU per 0.2 mL* For ...
-
PRINCIPAL DISPLAY PANEL - 0.3 mL Syringe Label - 02060.3 mL - NDC 0069-0206-01 - Fragmin® (dalteparin sodium) Injection, USP - 7,500 IU per 0.3 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.3 mL Syringe Blister Pack Label - 02060.3 mL Single-dose prefilled syringe - NDC 0069-0206-01 - Fragmin® (dalteparin sodium) Injection, USP - 7,500 IU per 0.3 mL - For subcutaneous injection - Rx only - PAA194312
-
PRINCIPAL DISPLAY PANEL - 0.3 mL Syringe Carton - 0206NDC 0069-0206-02 - Contains 10 of NDC 0069-0206-01 - 10 x 0.3 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 7,500 IU per 0.3 mL* For ...
-
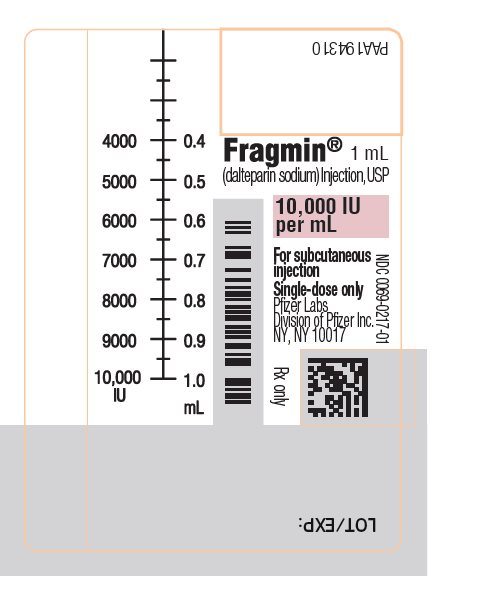
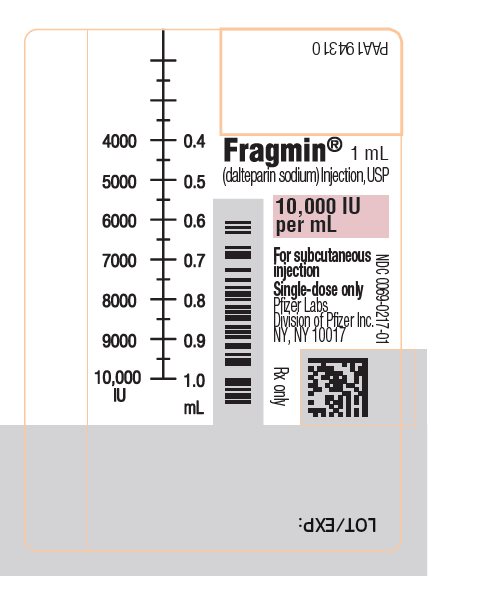
PRINCIPAL DISPLAY PANEL - 1 mL Syringe Label - 0217PAA194310 - Fragmin® 1 mL - (dalteparin sodium) Injection, USP - 10,000 IU - per mL - NDC 0069-0217-01 - For subcutaneous - injection - Single-dose only. Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - NDC ...
-
PRINCIPAL DISPLAY PANEL - 1 mL Syringe Blister Pack Label - 02171 mL Single-dose graduated syringe - NDC 0069-0217-01 - Fragmin® (dalteparin sodium) Injection, USP - 10,000 IU per mL - For subcutaneous injection - Rx only - PAA194270
-
PRINCIPAL DISPLAY PANEL - 1 mL Syringe Carton - 0217NDC 0069-0217-02 - Contains 10 of NDC 0069-0217-01 - 10 x 1 mL Single-dose graduated syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 10,000 IU per mL* For ...
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label - 02200.5 mL NDC 0069-0220-01 - Fragmin® (dalteparin sodium) Injection, USP - 12,500 IU per 0.5 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Blister Pack Label - 02200.5 mL Single-dose prefilled syringe - NDC 0069-0220-01 - Fragmin® (dalteparin sodium) Injection, USP - 12,500 IU per 0.5 mL - For subcutaneous injection - Rx only - PAA194307
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton - 0220NDC 0069-0220-02 - Contains 10 of NDC 0069-0220-01 - 10 x 0.5 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 12,500 IU per 0.5 mL* For ...
-
PRINCIPAL DISPLAY PANEL - 0.6 mL Syringe Label-02230.6 mL NDC 0069-0223-01 - Fragmin® (dalteparin sodium) Injection, USP - 15,000 IU per 0.6 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.6 mL Syringe Blister Pack Label - 02230.6 mL Single-dose prefilled syringe - NDC 0069-0223-01 - Fragmin® (dalteparin sodium) Injection, USP - 15,000 IU per 0.6 mL - For subcutaneous injection - Rx only - PAA194305
-
PRINCIPAL DISPLAY PANEL - 0.6 mL Syringe Carton - 0223NDC 0069-0223-02 - Contains 10 of NDC 0069-0223-01 - 10 x 0.6 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection,USP - 15,000 IU per 0.6 mL* For ...
-
PRINCIPAL DISPLAY PANEL - 0.72 mL Syringe Label - 02280.72 mL - NDC 0069-0228-01 - Fragmin® (dalteparin sodium) Injection, USP - 18,000 IU per 0.72 mL - For subcutaneous injection - Single-dose only - Pfizer Labs - Division of Pfizer Inc. NY, NY 10017 - Rx ...
-
PRINCIPAL DISPLAY PANEL - 0.72 mL Syringe Blister Pack Label - 02280.72 mL Single-dose prefilled syringe - NDC 0069-0228-01 - Fragmin® (dalteparin sodium) Injection, USP - 18,000 IU per 0.72 mL - For subcutaneous injection - Rx only - PAA194308
-
PRINCIPAL DISPLAY PANEL - 0.72 mL Syringe Carton - 0228NDC 0069-0228-02 - Contains 10 of NDC 0069-0228-01 - 10 x 0.72 mL Single-dose prefilled syringes, preassembled with needle guards - Fragmin® (dalteparin sodium) Injection, USP - 18,000 IU per 0.72 ...
-
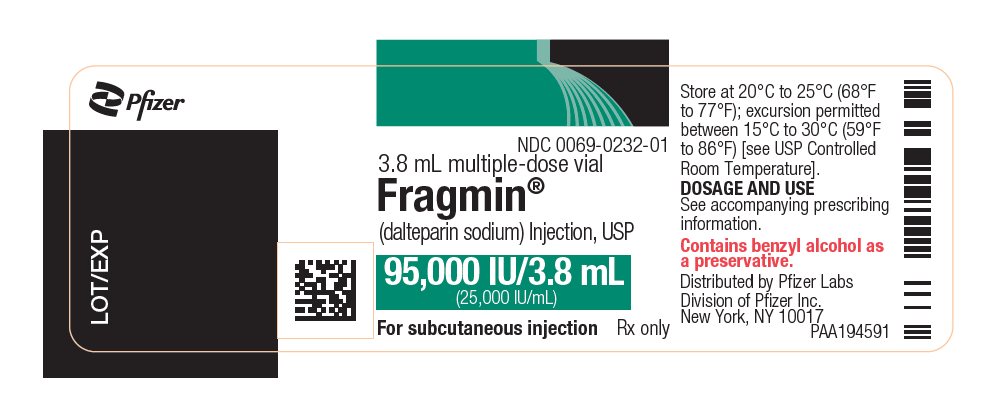
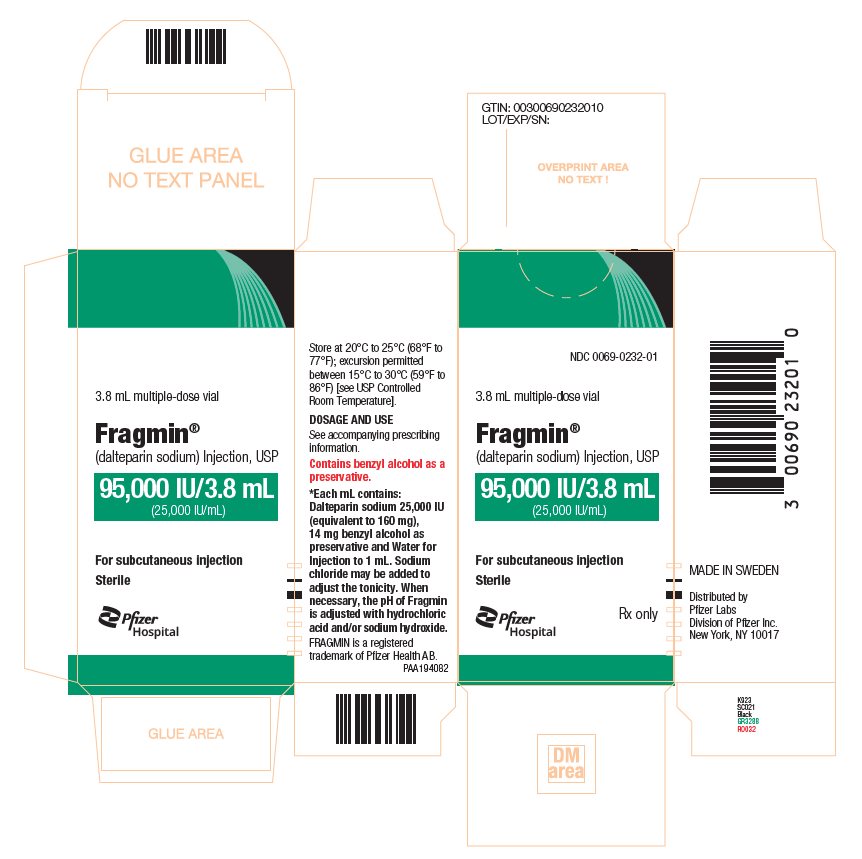
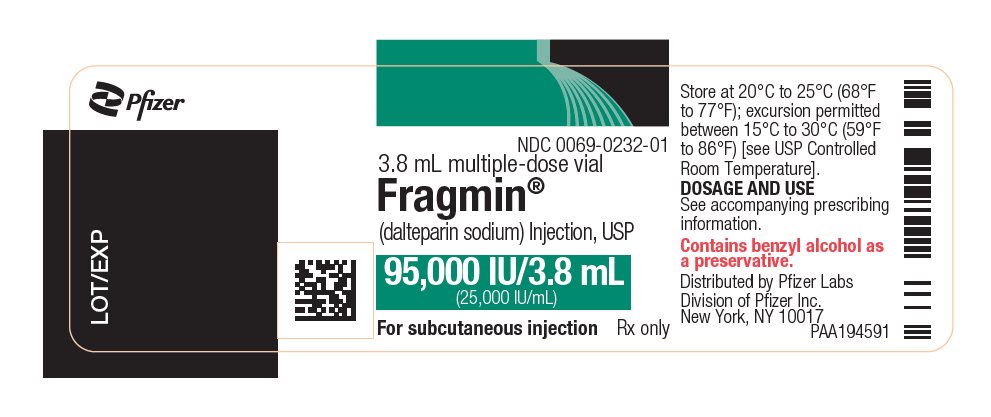
PRINCIPAL DISPLAY PANEL - 95,000 IU Vial Label - 0232NDC 0069-0232-01 - 3.8 mL multiple-dose vial - Fragmin® (dalteparin sodium) Injection, USP - 95,000 IU/3.8 mL - (25,000 IU/mL) For subcutaneous injection - Rx only
-
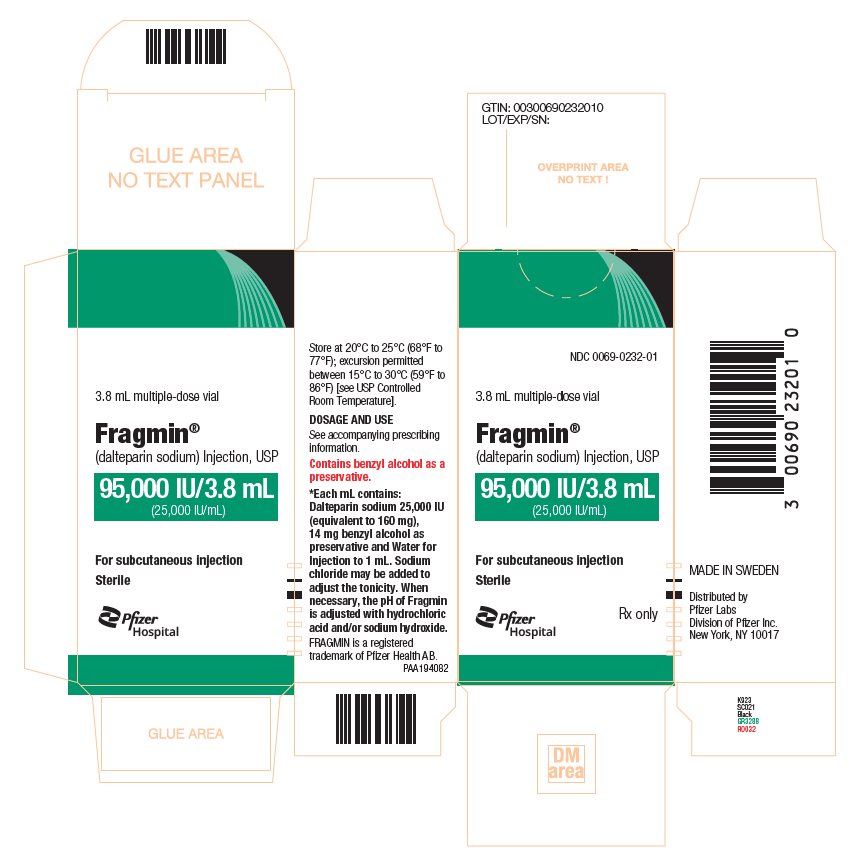
PRINCIPAL DISPLAY PANEL - 95,000 IU Vial Carton - 0232NDC 0069-0232-01 - 3.8 mL multiple-dose vial - Fragmin® (dalteparin sodium) Injection, USP - 95,000 IU/3.8 mL - (25,000 IU/mL) For subcutaneous injection - Sterile - Pfizer Injectables - Rx only
-
PRINCIPAL DISPLAY PANEL - 10,000 IU Vial Label - 0253 NDC 0069-0253-01 - 4 mL single-dose vial - Fragmin® (dalteparin sodium) Injection, USP - 10,000 IU/4 mL - (2,500 IU/mL) For subcutaneous injection - Rx only
-
PRINCIPAL DISPLAY PANEL - 10,000 IU Vial Carton - 0253 NDC 0069-0253-10 - Ten 4 mL single-dose vials - Fragmin® (dalteparin sodium) Injection, USP - 10,000 IU/4 mL - (2,500 IU/mL) For subcutaneous injection - Sterile - Rx only - Pfizer - Hospital
-
INGREDIENTS AND APPEARANCEProduct Information