Label: TESTOSTERONE gel, metered
- NDC Code(s): 0591-2363-60
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TESTOSTERONE GEL safely and effectively. See full prescribing information for TESTOSTERONE GEL. TESTOSTERONE gel, for topical ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SECONDARY EXPOSURE TO TESTOSTERONE
- Virilization has been reported in children who were secondarily exposed to testosterone gel [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
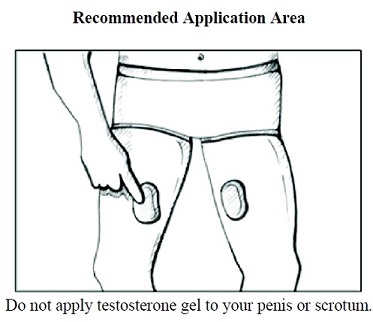
- Children should avoid contact with unwashed or unclothed application sites in men using testosterone gel [see Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
- Healthcare providers should advise patients to strictly adhere to recommended instructions for use [see Dosage and Administration (2.2), Warnings and Precautions (5.2), and Patient Counseling Information (17)].
-
1 INDICATIONS AND USAGE
Testosterone topical gel is indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone: Primary hypogonadism (congenital or ...
-
2 DOSAGE AND ADMINISTRATION
Prior to initiating testosterone gel, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least 2 separate days and ...
-
3 DOSAGE FORMS AND STRENGTHS
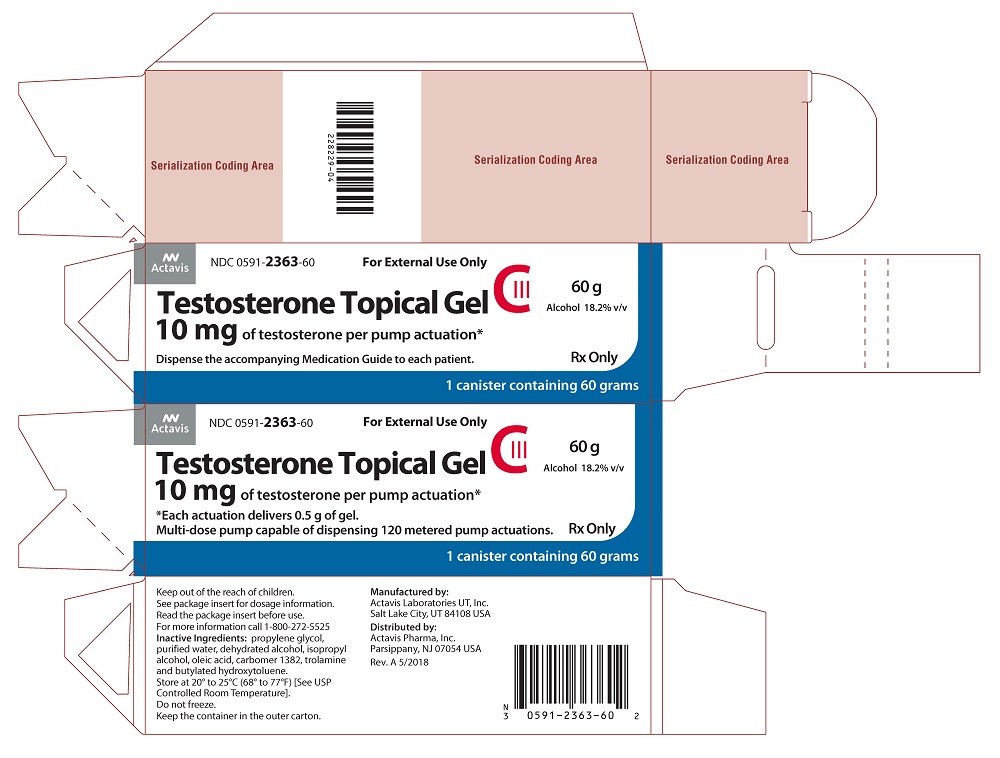
Testosterone gel for topical use only, is supplied in a metered-dose pump. One (1) pump actuation delivers 10 mg of testosterone.
-
4 CONTRAINDICATIONS
Testosterone gel is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)] ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer - Patients with BPH treated with androgens are at an increased risk of worsening of signs and symptoms ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Insulin - Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Testosterone gel is contraindicated in pregnant women. Testosterone is teratogenic and may cause fetal harm based on data from animal studies and its mechanism of ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Testosterone gel contains testosterone, a Schedule III controlled substance in the Controlled Substances Act. 9.2 Abuse - Drug abuse is intentional ...
-
10 OVERDOSAGE
There is a single report of acute overdosage after parenteral administration of an approved testosterone product in the literature. This subject had serum testosterone concentrations of up to ...
-
11 DESCRIPTION
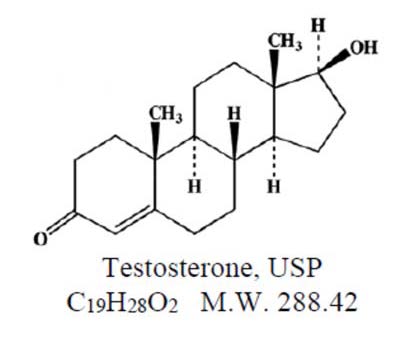
Testosterone Topical Gel is a clear, colorless, odorless, gel containing testosterone, USP. Testosterone Topical Gel is available in a metered-dose pump. Each pump actuation provides 10 mg of ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for the ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, implant-induced ...
-
14 CLINICAL STUDIES
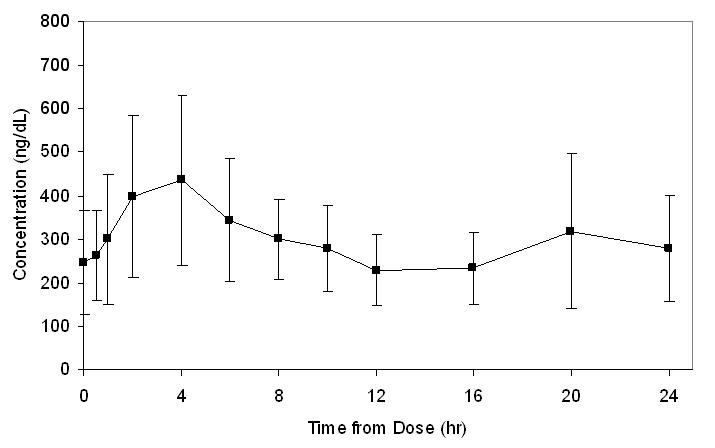
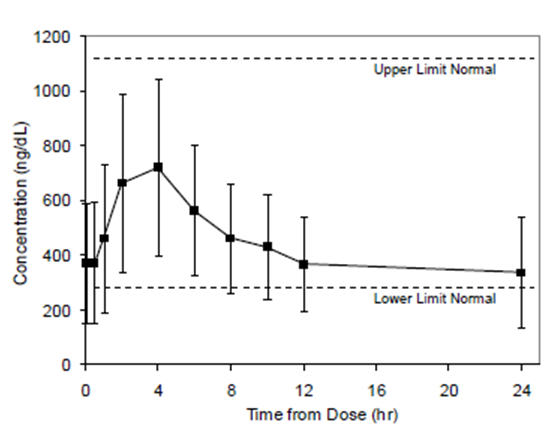
14.1 Clinical Study in Hypogonadal Males - Testosterone gel was evaluated in a multicenter, 90-day open-label, non-comparative trial of 149 hypogonadal males with body mass index (BMI ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Testosterone Topical Gel is supplied in 60 g canisters with a metered dose pump that delivers 10 mg of testosterone, USP per complete pump actuation. The metered dose pump is capable of dispensing ...
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved Medication Guide. Patients should be informed of the following information: 17.1 - Use in Men with Known or Suspected Prostate or Breast Cancer - Men with known or suspected ...
-
MEDICATION GUIDETestosterone (tes tos' ter one) CIII - Topical Gel - What is the most important information I should know about testosterone gel? 1. Testosterone gel can transfer from your body to ...
-
INSTRUCTIONS FOR USETestosterone (tes tos' ter one) CIII - Topical Gel - Read this Instructions for Use for testosterone gel before you start using it and each time you get a refill. There may be new information ...
-
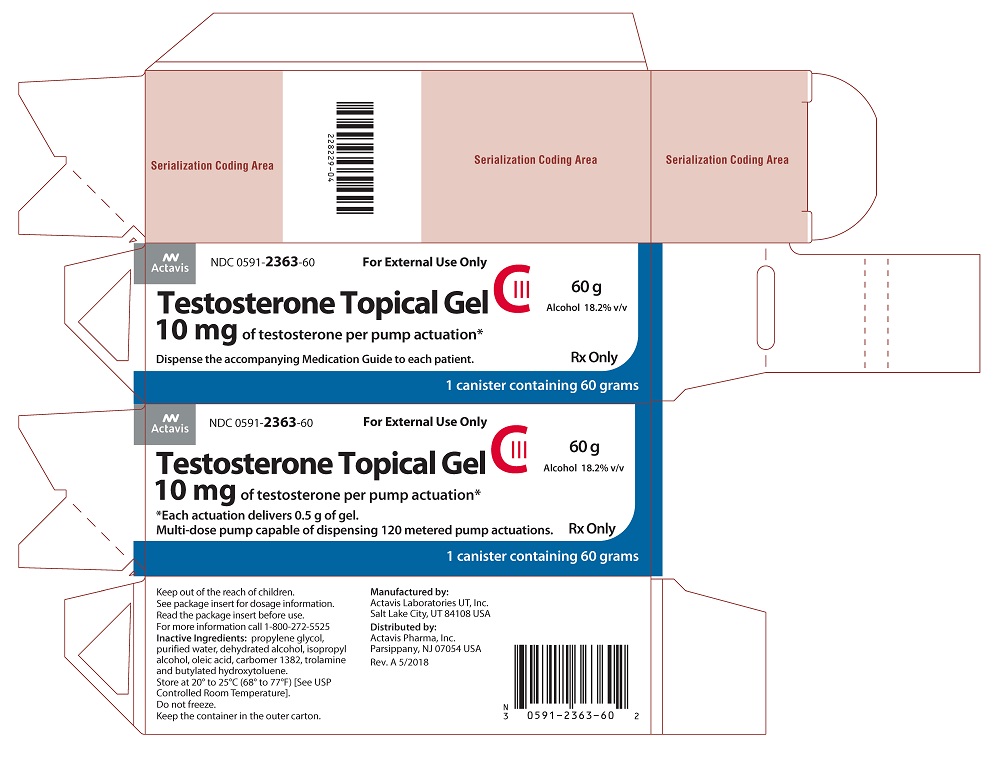
PACKAGE LABEL PRINCIPAL DISPLAY PANELNDC 0591-2363-60 - For External Use Only - Testosterone Topical Gel CIII - 10 mg of testosterone per pump actuation* 60 g - Alcohol 18.2% v/v - *Each actuation delivers 0.5 g of gel. Multi-dose pump capable ...
-
INGREDIENTS AND APPEARANCEProduct Information