Label: FOLOTYN- pralatrexate injection
- NDC Code(s): 72893-003-01, 72893-005-01
- Packager: Acrotech Biopharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FOLOTYN® safely and effectively. See full prescribing information for FOLOTYN. FOLOTYN (pralatrexate injection), for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFOLOTYN is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is approved under accelerated approval based on overall response ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosing Information - Pretreatment Vitamin Supplementation - Folic Acid - Instruct patients to take folic acid 1 to 1.25 mg orally once daily beginning 10 days before the first dose ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 40 mg/2 mL (20 mg/mL) and 20 mg/mL clear yellow sterile solution in single-dose vial
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - FOLOTYN can cause myelosuppression, manifested by thrombocytopenia, neutropenia, and/or anemia. Administer vitamin B12 and instruct patients to take folic acid to reduce ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.1)] Mucositis [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on FOLOTYN - Coadministration of FOLOTYN with probenecid increased pralatrexate plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], FOLOTYN can cause fetal harm when administered to a pregnant ...
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage of FOLOTYN. If an overdose occurs, general supportive measures should be instituted as deemed necessary by the treating ...
-
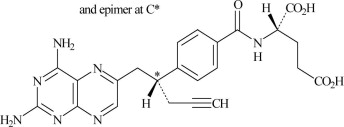
11 DESCRIPTIONPralatrexate is a dihydrofolate reductase inhibitor. Pralatrexate has the chemical name (2S)-2-[[4-[(1RS)-1-[(2, 4-diaminopteridin-6-yl)methyl]but-3- ynyl]benzoyl]amino]pentanedioic acid. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pralatrexate is a folate analog metabolic inhibitor that competitively inhibits dihydrofolate reductase. It is also a competitive inhibitor for polyglutamylation by the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies have not been performed with pralatrexate. Mutagenesis - Pralatrexate did not cause ...
-
14 CLINICAL STUDIESThe efficacy of FOLOTYN was evaluated in Study PDX-008, an open-label, single-arm, multi-center, international trial that enrolled patients with relapsed or refractory PTCL. One hundred and eleven ...
-
15 REFERENCES1. “OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFOLOTYN is available in clear glass single-dose vials containing pralatrexate at a concentration of 20 mg/mL as a preservative-free, sterile, clear yellow solution individually packaged for ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Folic Acid and Vitamin B12 Supplementation - Advise patients treated with FOLOTYN to take folic acid and ...
-
SPL PATIENT PACKAGE INSERT SECTIONPatient Information - FOLOTYN®(FOH-loh-tin) (pralatrexate injection) What is FOLOTYN? FOLOTYN is a prescription used to treat people with a type of cancer called peripheral T-cell lymphoma ...
-
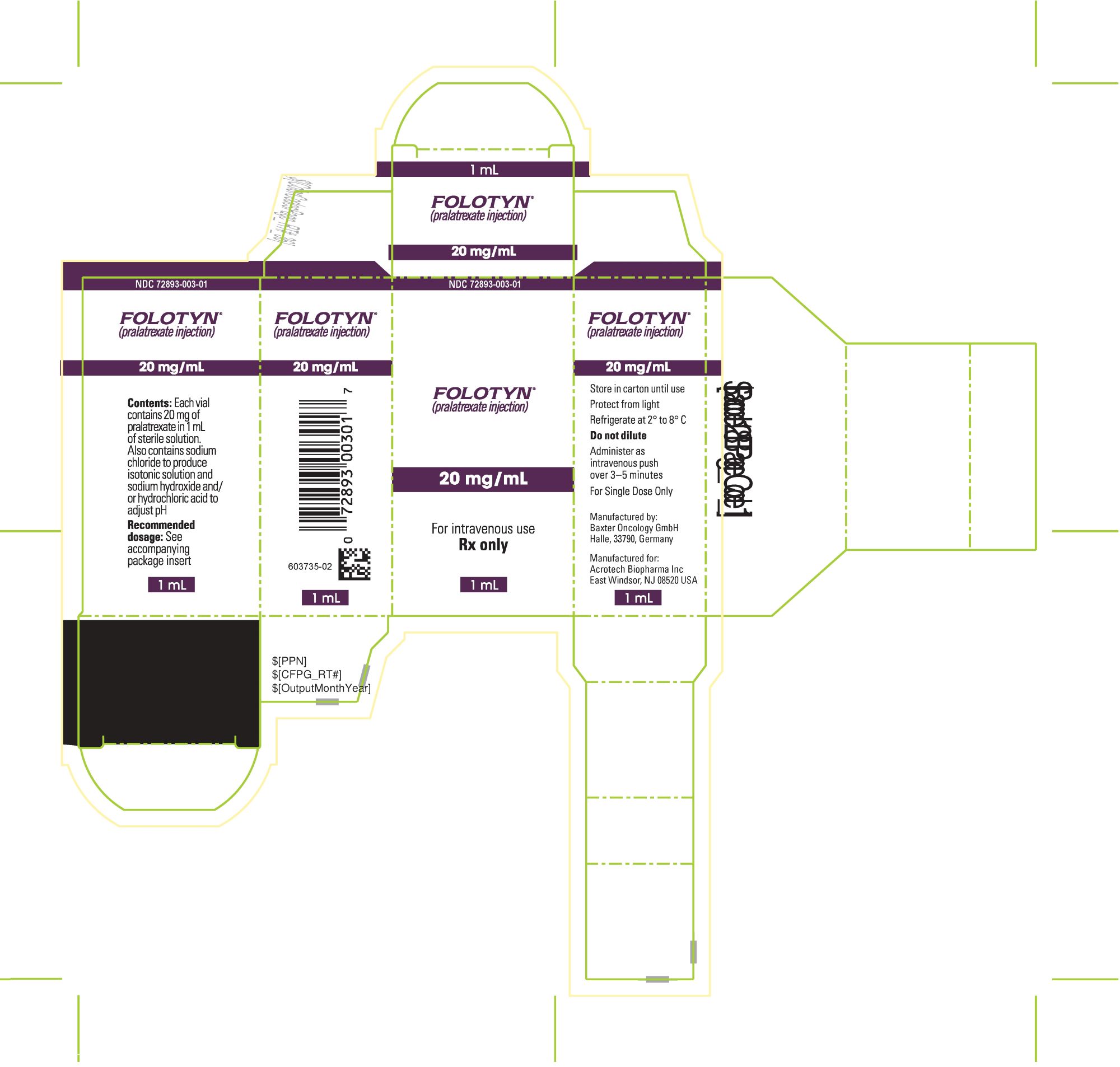
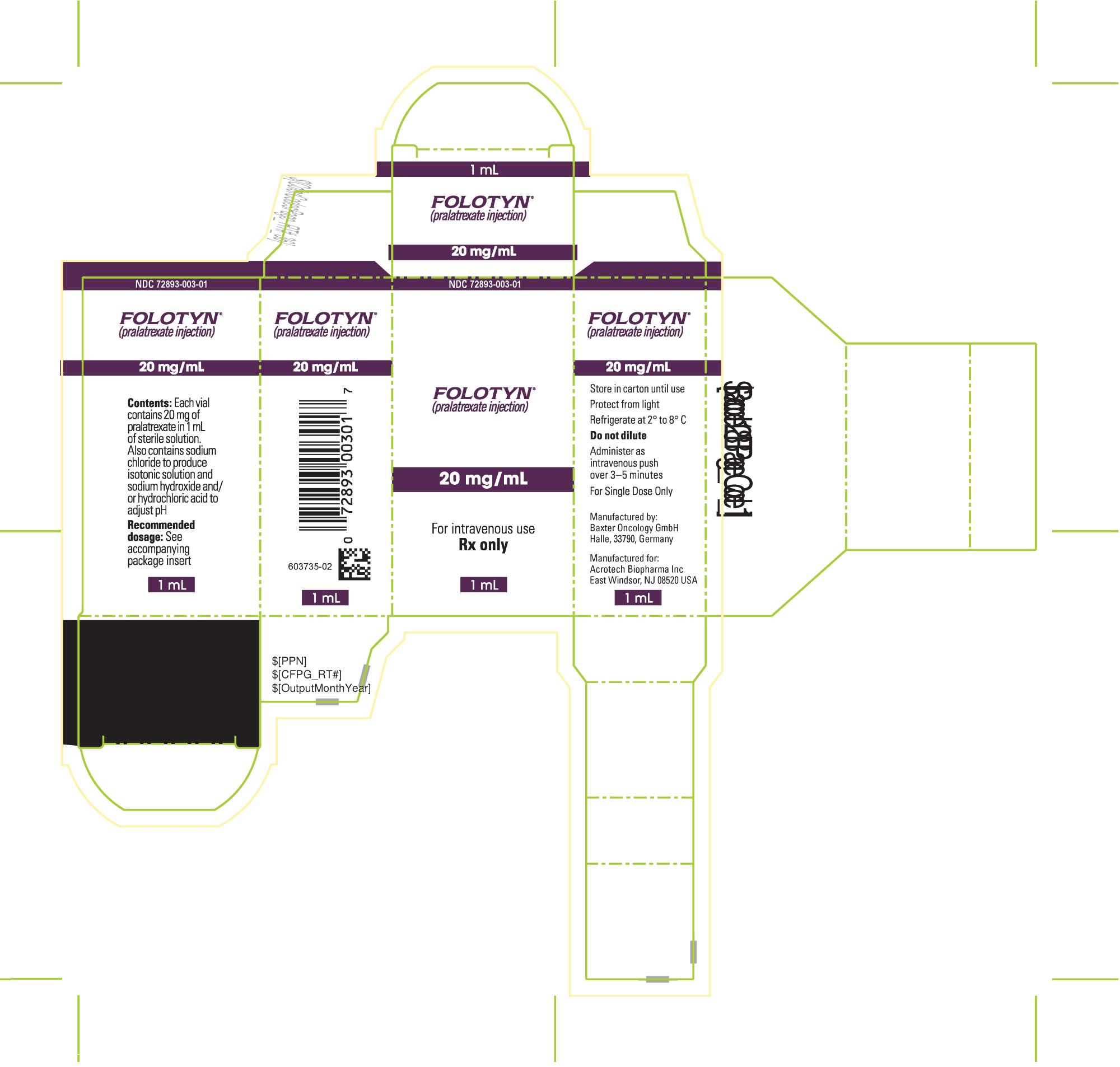
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPACKAGE CARTON - FOLOTYN 20 mg/1 mL Vial - NDC 72893-003-01 - FOLOTYN® (pralatrexate injection) 20 mg/mL - For intravenous use - Rx only - 1 mL
-
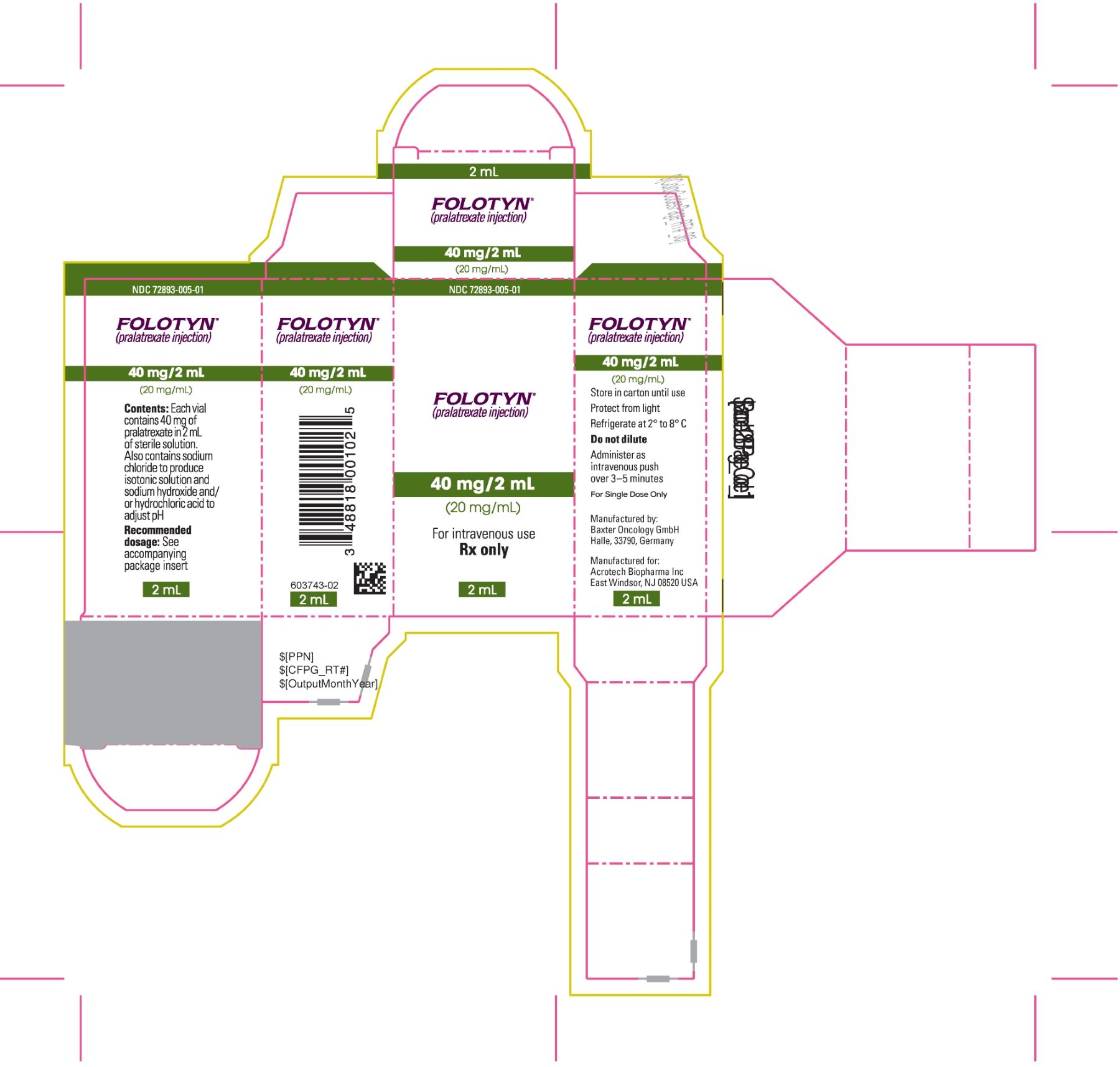
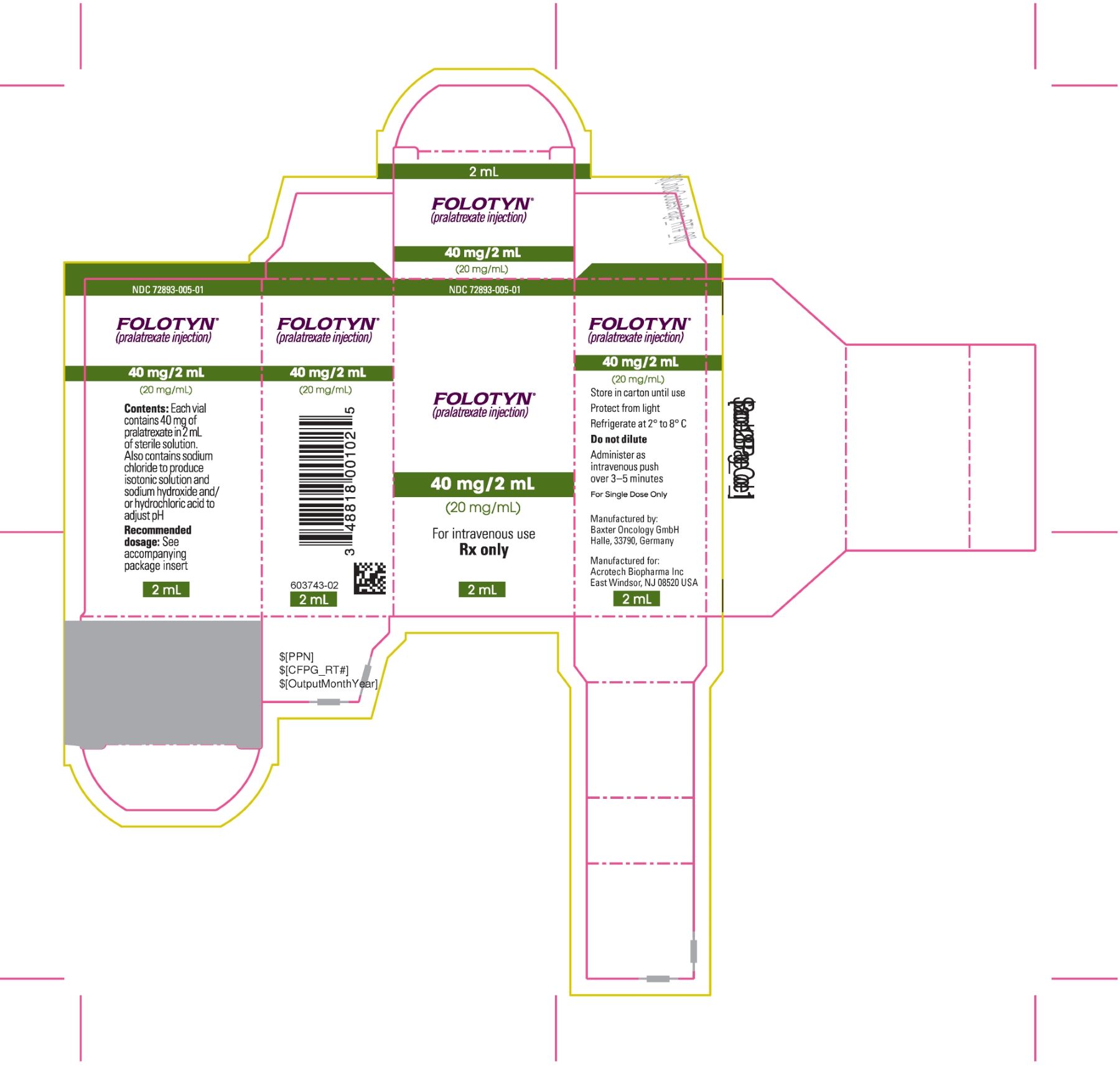
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPACKAGE CARTON - FOLOTYN 40 mg/2 mL Vial - NDC 72893-005-01 - FOLOTYN® (pralatrexate injection) 40 mg/2 mL - (20 mg/mL) For intravenous use - Rx only - 2 mL
-
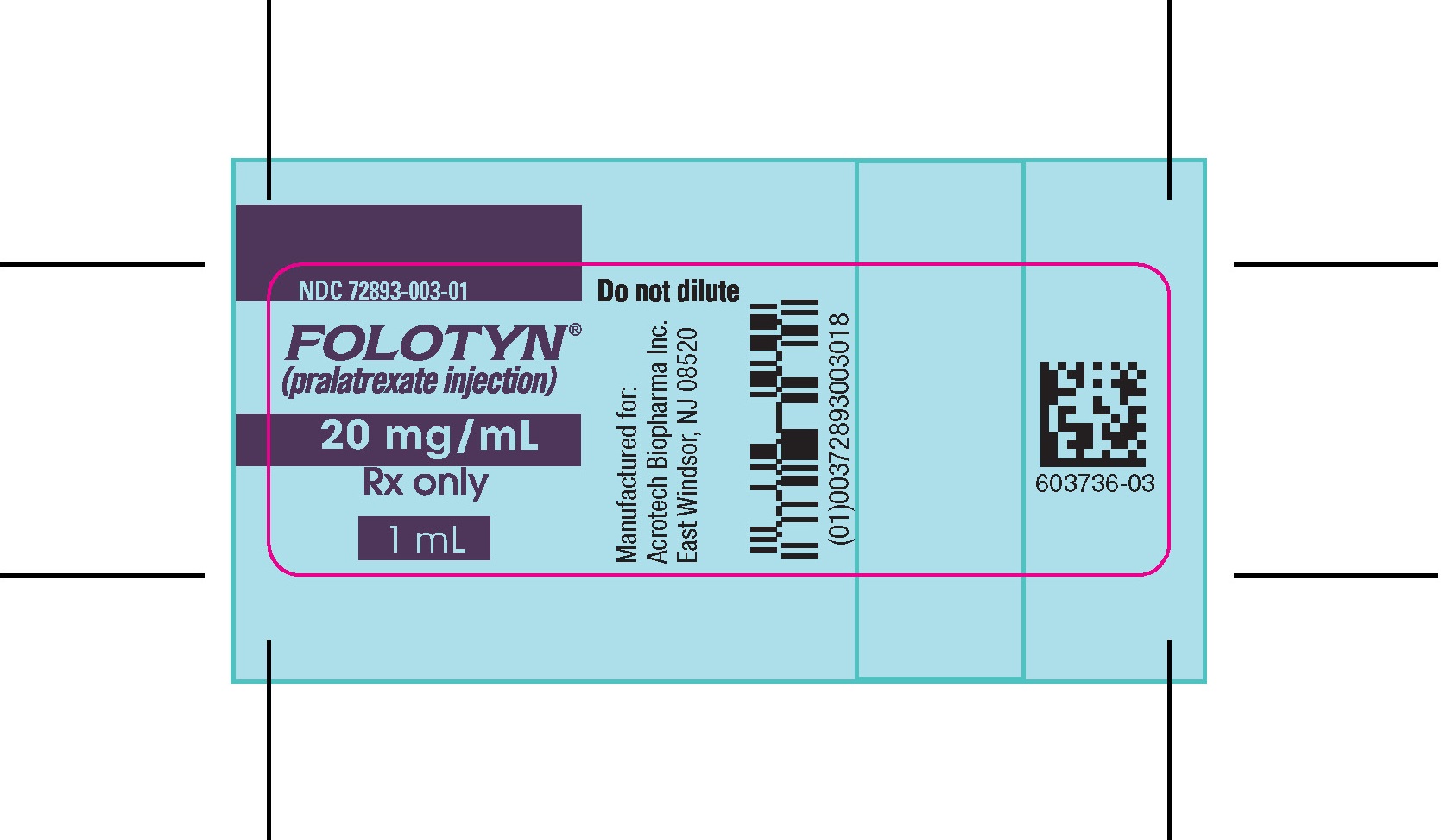
PACKAGE LABEL.PRINCIPAL DISPLAY PANELVIAL – FOLOTYN 20 mg/1 ml - NDC 72893-003-01 - FOLOTYN® (pralatrexate injection) 20 mg/mL - Rx only - 1 mL
-
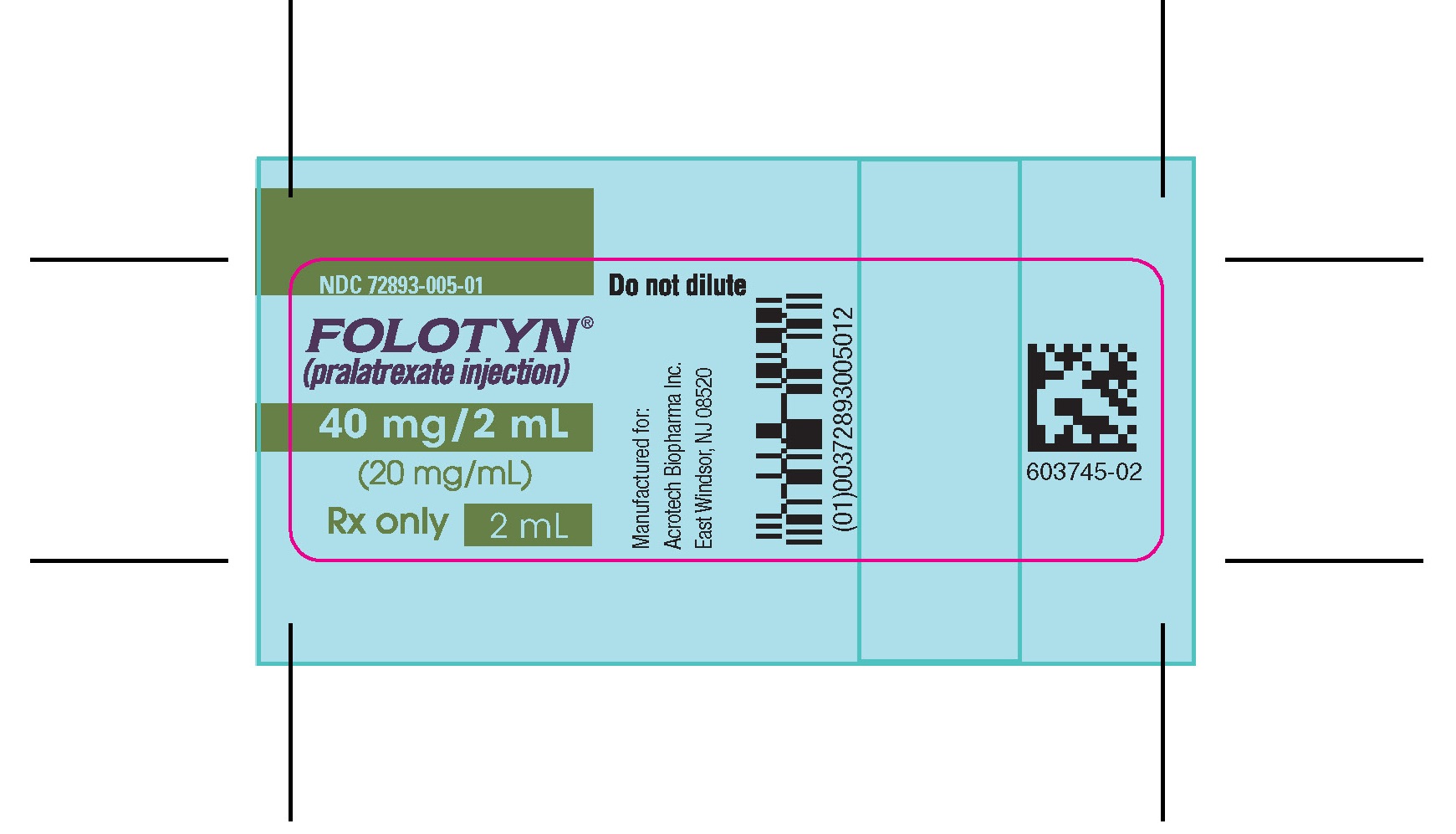
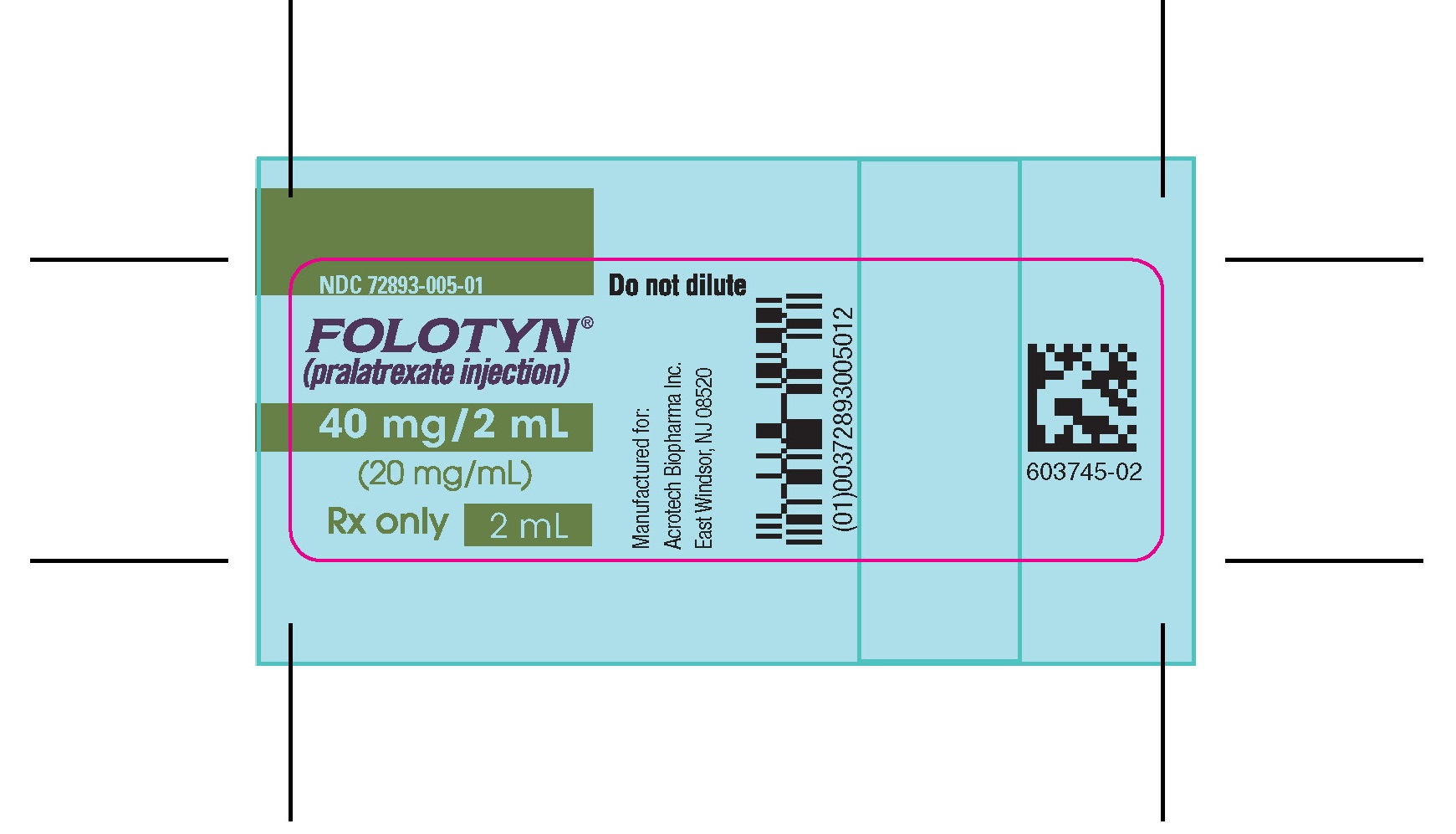
PACKAGE LABEL.PRINCIPAL DISPLAY PANELVIAL – FOLOTYN 40 mg/2 ml - NDC 72893-005-01 - FOLOTYN® (pralatrexate injection) 40 mg/2 mL - (20 mg/mL) Rx only - 2 mL
-
INGREDIENTS AND APPEARANCEProduct Information