Label: FLUOPAR- fluocinonide, dimethicone kit
- NDC Code(s): 45802-151-94, 59088-333-08, 59088-754-00
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
1 INDICATIONS AND USAGE
These highlights do not include all the information needed to use Fluocinonide Cream USP, 0.1% safely and effectively. See full prescribing information for Fluocinonide Cream USP, 0.1%.
Fluocinonide Cream USP, 0.1%
For topical use
Initial U.S. Approval: 1971
HIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use Fluocinonide Cream USP, 0.1% safely and effectively. See full prescribing information for ...
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Fluocinonide Cream USP, 0.1% is not for ophthalmic, oral, or intravaginal use. Psoriasis: apply a thin layer once or twice daily to the affected skin areas. Atopic ...
-
3 DOSAGE FORMS AND STRENGTHS
Cream, 0.1% (3) Each gram of Fluocinonide Cream USP, 0.1% contains 1 mg of fluocinonide in a white to off-white cream base.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS•Fluocinonide Cream USP, 0.1% has been shown to suppress the HPA axis. Systemic absorption of Fluocinonide Cream USP, 0.1% may produce reversible hypothalamic-pituitary-adrenal (HPA) axis ...
-
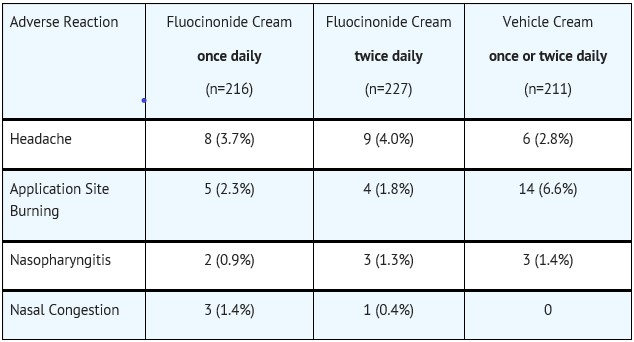
6 ADVERSE REACTIONSThe most commonly reported adverse reactions (≥1%) were headache, application site burning, nasopharyngitis, and nasal congestion. To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratorgenic Effects: Pregnancy Category C - There are no adequate and well-controlled studies in pregnant women. Therefore, Fluocinonide Cream USP, 0.1% should be used during ...
-
10 OVERDOSAGETopically applied Fluocinonide Cream USP, 0.1% can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)].
-
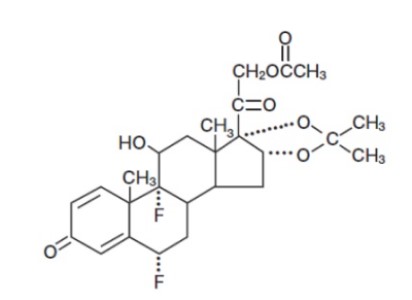
11 DESCRIPTIONFluocinonide Cream USP, 0.1% contains fluocinonide, a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of Fluocinonide Cream ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of fluocinonide cream, 0.1% because of severe ...
-
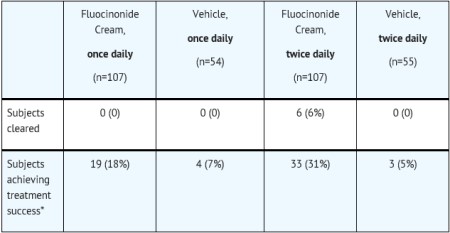
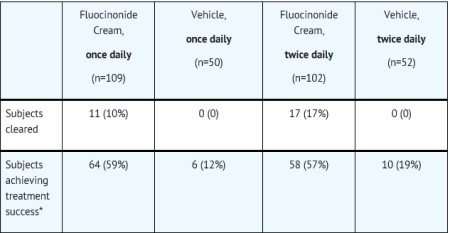
14 CLINICAL STUDIESTwo adequate and well-controlled efficacy and safety studies of fluocinonide cream, 0.1% have been completed, one in adult subjects with plaque-type psoriasis (Table 2), and one in adult subjects ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFluocinonide Cream USP, 0.1% is white to off-white in color and is supplied in tubes as follows: 30 g (NDC 45802- 151-94) 60 g (NDC 45802 ...
-
STORAGE AND HANDLINGStore at controlled room temperature: 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Keep the tube tightly closed.
-
17 PATIENT COUNSELING INFORMATION[See FDA-approved patient labeling (Patient Information)] Patients using Fluocinonide Cream USP, 0.1% should receive the following information and instructions. This information is intended to aid ...
-
PATIENT INFORMATIONFluocinonide Cream USP, 0.1% Important: For skin use only. Do not get Fluocinonide Cream USP, 0.1% in your eyes, mouth, or vagina. Not for use on the face, groin, or underarms ...
-
Skin Repair Complex (Dimethicone Cream 5%)Drug Facts
-
Active ingredient
Dimethicone 5.0%
-
Purpose
Skin Protectant
-
Uses
■ for the treatment and/or prevention of diaper rash - ■ temporarily protects and helps relieve chapped or cracked skin
-
Warnings
For external use only - Do not use on ■ deep or puncture wounds ■ animal bites ■ serious burns - When using this product ■ do not get into eyes - Stop ...
-
Directions
■ apply cream liberally as needed
-
Other information
■ protect from freezing ■ avoid excessive heat
-
Inactive ingredients
Aleurites moluccana seed oil, Aloe barbadensis (Aloe vera) leaf juice, butylene glycol, caprylyl glycol, Carthamus tinctorius (safflower) seed oil, cetyl alcohol, chlorphenesin, dimethicone ...
-
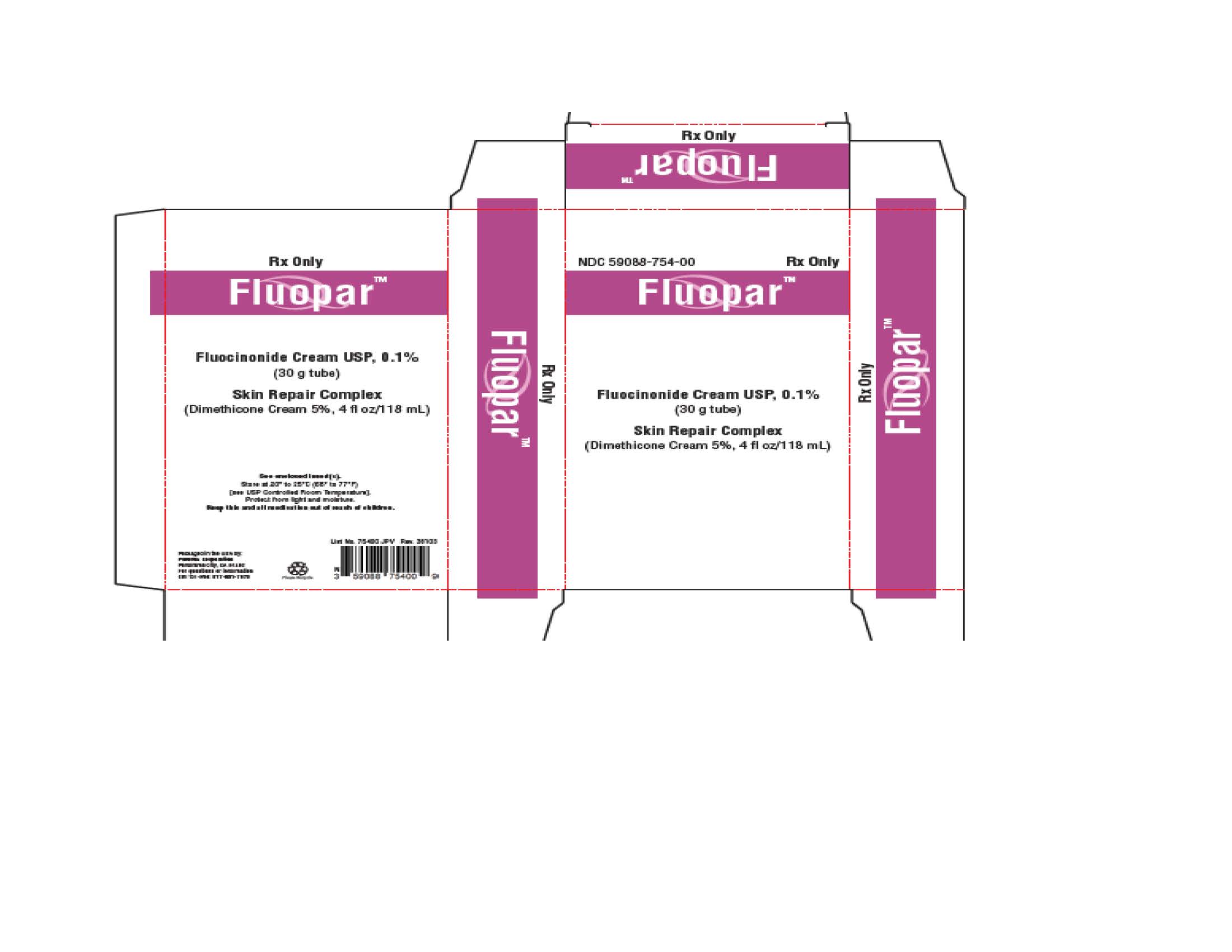
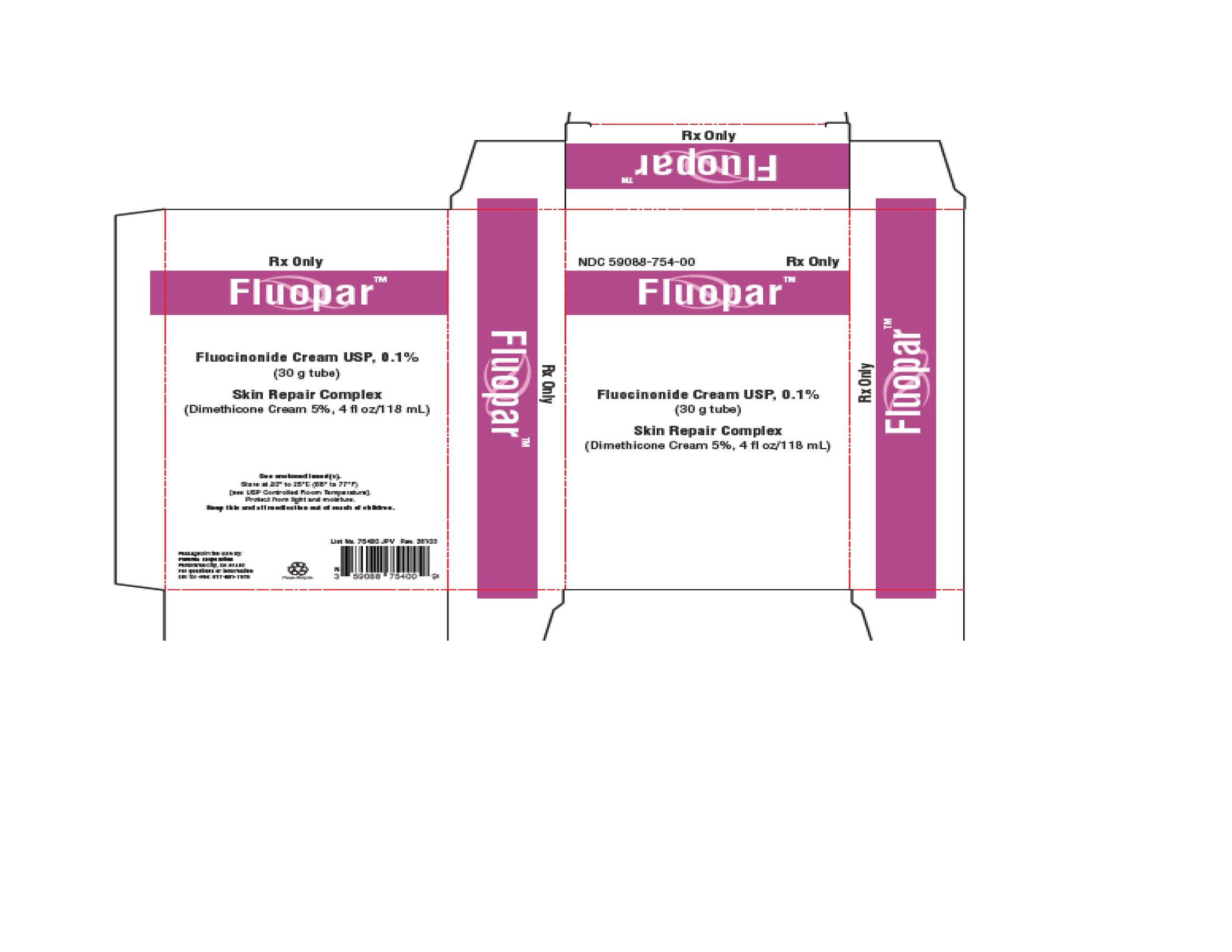
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 30 g CartonRx Only - Fluocinonide Cream USP, 0.1% For Topical Use Only. Not for Ophthalmic, Oral, or Intravaginal Use. NET WT 30 g
-
PRINCIPAL DISPLAY PANEL
NDC 59088-746-00 - DermacinRx® Fluorpar™ [Fluocinonide Cream USP, 0.1% and Skin Repair Complex (Dimethicone Cream 5%)] Rx only - Packaged in the USA by ...
-
INGREDIENTS AND APPEARANCEProduct Information