Label: FLUOCINOL PAK- fluocinonide kit
- NDC Code(s): 45802-151-94, 59088-755-00
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONFluocinol Pak - (Fluocinonide Cream USP, Sterile Alcohol Prep Pads, Gauze Pads, Silicone Tape) These highlights do not include all the information needed to use Fluocinonide Cream USP, 0.1 ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - Fluocinonide Cream USP, 0.1%, is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses in patients 12 years of age or ...
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Fluocinonide Cream USP, 0.1% is not for ophthalmic, oral, or intravaginal use. For psoriasis, apply a thin layer of Fluocinonide Cream USP, 0.1% once or twice daily to the ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 0.1%. Each gram of Fluocinonide Cream USP, 0.1% contains 1 mg of fluocinonide in a white to off-white cream base.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Effect on Endocrine System - Systemic absorption of topical corticosteroids, including Fluocinonide Cream USP, 0.1%, can produce reversible hypothalamic-pituitary-adrenal (HPA) axis ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratorgenic Effects: Pregnancy Category C - There are no adequate and well-controlled studies in pregnant women. Therefore, Fluocinonide Cream USP, 0.1% should be used ...
-
10 OVERDOSAGETopically applied Fluocinonide Cream USP, 0.1% can be absorbed in sufficient amounts to produce systemic effects [ see Warnings and Precautions (5.1)].
-
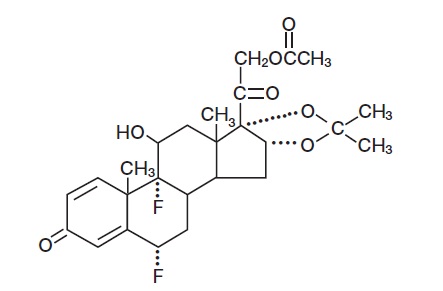
11 DESCRIPTIONFluocinonide Cream USP, 0.1% contains fluocinonide, a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of Fluocinonide Cream ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of fluocinonide cream, 0.1% because of severe ...
-
14 CLINICAL STUDIESTwo adequate and well-controlled efficacy and safety studies of fluocinonide cream, 0.1% have been completed, one in adult subjects with plaque-type psoriasis (Table 2), and one in adult subjects ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFluocinonide Cream USP, 0.1% is white to off-white in color and is supplied in tubes as follows: 30 g (NDC 45802- 151-94) Store at controlled room temperature: 20-25°C (68-77°F) [see USP ...
-
17 PATIENT COUNSELING INFORMATION[See FDA-approved patient labeling (Patient Information)] Patients using Fluocinonide Cream USP, 0.1% should receive the following information and instructions. This information is intended to ...
-
PATIENT INFORMATIONFluocinonide Cream USP, 0.1% Important: For skin use only. Do not get Fluocinonide Cream USP, 0.1% in your eyes, mouth, or vagina. Not for use on the face, groin, or underarms. Read the ...
-
Fluocinol Pak
TM
Packaged in the USA by: PureTek Corporation - Panorama City, CA 91402 - For questions or information - call toll-free: 877-921-7873 - Rev. 38106
-
INGREDIENTS AND APPEARANCEProduct Information