Label: FIRDAPSE- amifampridine phosphate tablet
- NDC Code(s): 69616-211-03, 69616-211-04, 69616-211-06, 69616-211-08
- Packager: Catalyst Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FIRDAPSE® safely and effectively. See full prescribing information for FIRDAPSE®. FIRDAPSE® (amifampridine) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
FIRDAPSE® is indicated for the treatment of Lambert-Eaton myasthenic syndrome (LEMS) in adults and pediatric patients 6 years of age and older.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information - The recommended oral dosage of FIRDAPSE for adults and pediatric patients 6 years of age and older is included in Table 1. For pediatric patients, the recommended dosing ...
-
3 DOSAGE FORMS AND STRENGTHS
FIRDAPSE tablets contain 10 mg amifampridine and are white to off-white, round, and functionally scored. Each tablet is debossed on the non-scored side with “CATALYST” and on the scored side with ...
-
4 CONTRAINDICATIONS
FIRDAPSE is contraindicated in patients with: A history of seizures [see Warnings and Precautions (5.1)] Hypersensitivity to amifampridine phosphate or another aminopyridine [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Seizures - FIRDAPSE can cause seizures. Seizures have been observed in patients without a history of seizures taking FIRDAPSE at the recommended doses, at various times after initiation of ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Seizures [see Warnings and Precautions (5.1)] Hypersensitivity [see Warnings and Precautions (5.2)] 6.1 ...
-
7 DRUG INTERACTIONS
7.1 Drugs that Lower Seizure Threshold - The concomitant use of FIRDAPSE and drugs that lower seizure threshold may lead to an increased risk of seizures [see Warnings and Precautions (5.1)] ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to FIRDAPSE during pregnancy. Physicians are encouraged ...
-
10 OVERDOSAGE
Overdose with FIRDAPSE was not reported during clinical studies. In a case report, a 65-year-old patient with LEMS inadvertently received a total daily amifampridine dose of 360 mg/day ...
-
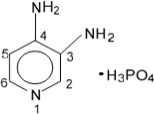
11 DESCRIPTION
The active ingredient of FIRDAPSE is amifampridine phosphate, which is a voltage-gated potassium channel blocker. Amifampridine phosphate is described chemically as 3,4-diaminopyridine phosphate ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism by which amifampridine exerts its therapeutic effect in LEMS patients has not been fully elucidated. Amifampridine is a broad-spectrum potassium channel ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - In a 104-week carcinogenicity study in rats, oral administration of amifampridine phosphate (0, 15, 48, or 105 ...
-
14 CLINICAL STUDIES
The efficacy of FIRDAPSE for the treatment of LEMS was demonstrated in two randomized, double-blind, placebo-controlled discontinuation studies. A total of 64 adults (age 21 to 88 years) with LEMS ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - FIRDAPSE 10 mg tablets are white to off white-round, and functionally scored. Each tablet is debossed on the non-scored side with “CATALYST” and on the scored side with “211 ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Risk of Seizures - Inform patients that FIRDAPSE can cause seizures ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration - Revised: 5/2024 - MEDICATION GUIDE - FIRDAPSE® (FIR-dapse) (amifampridine) tablets, for oral ...
-
INSTRUCTIONS FOR USEInstructions for Use - FIRDAPSE - (FIR-dapse) (amifampridine) tablets - for oral use - This Instructions for Use contains information on how to mix and use FIRDAPSE prepared suspension. The ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Carton
Rx Only - NDC 69616-211-06 - 120 Tablets Total - Provided in 12 Child-Resistant Blister Cards - Of 10 Tablets Each - FIRDAPSE® (amifampridine) Tablets - 10 mg per Tablet - Manufactured for: Catalyst ...
-
INGREDIENTS AND APPEARANCEProduct Information