Label: FIRAZYR- icatibant acetate injection, solution
- NDC Code(s): 54092-702-01, 54092-702-02, 54092-702-03
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FIRAZYR® (icatibant) safely and effectively. See full prescribing information for FIRAZYR. FIRAZYR (icatibant) Injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFIRAZYR® (icatibant) is indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older.
-
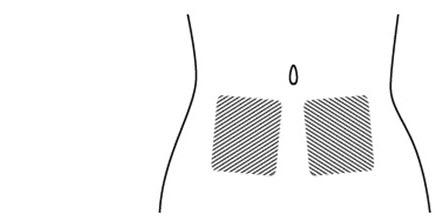
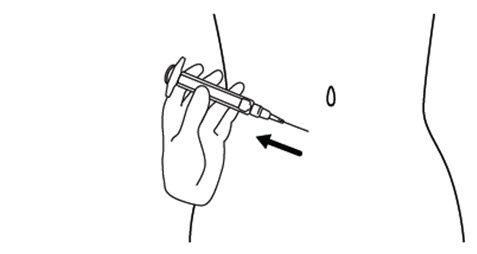
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - The recommended dose of FIRAZYR is 30 mg administered by subcutaneous (SC) injection in the abdominal area. Additional doses may be administered at intervals of at least ...
-
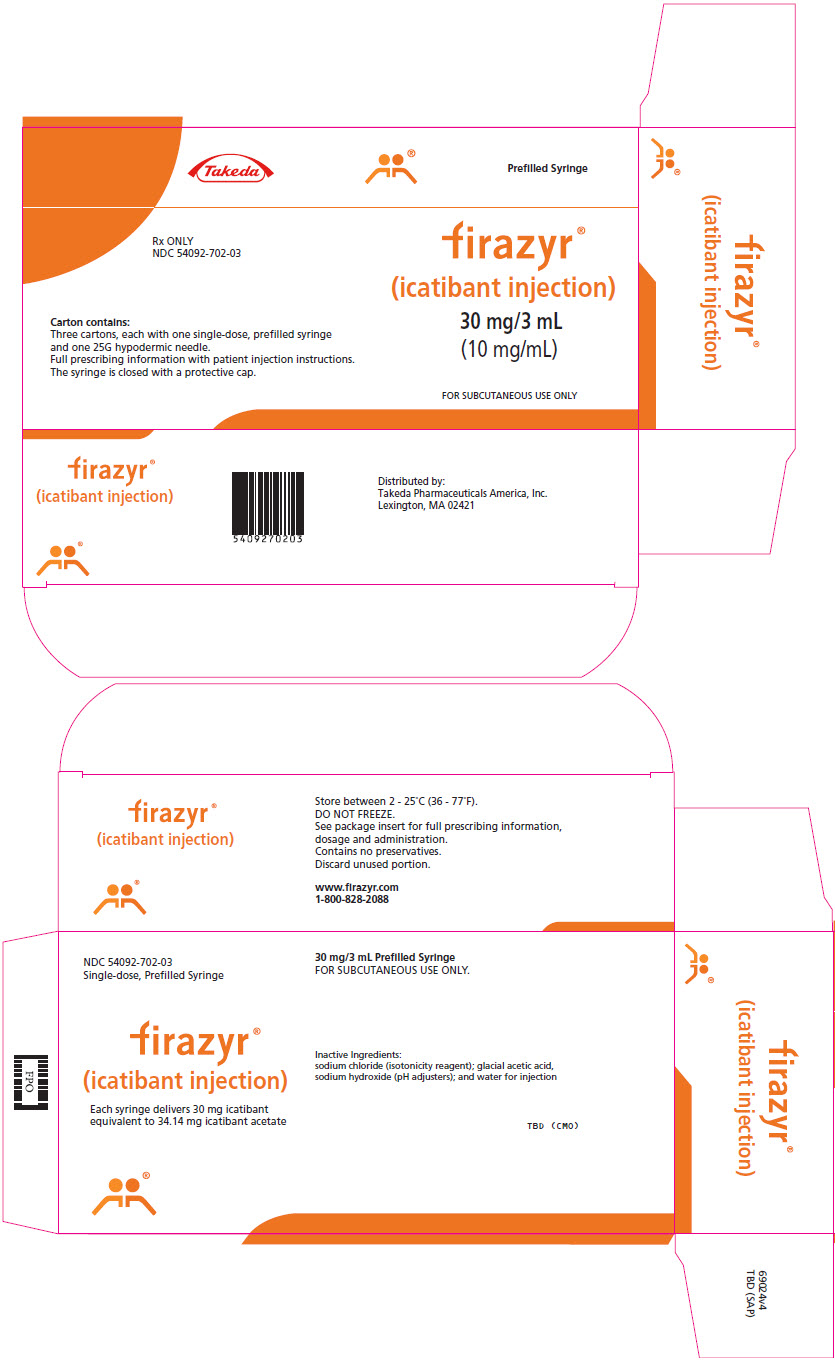
3 DOSAGE FORMS AND STRENGTHSFIRAZYR injection is supplied in a prefilled syringe delivering 30 mg icatibant. Each syringe delivers 3 mL solution with a concentration of 10 mg per mL.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Laryngeal Attacks - Given the potential for airway obstruction during acute laryngeal HAE attacks, patients should be advised to seek medical attention in an appropriate healthcare facility ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - The safety of icatibant was evaluated in three controlled trials that included 223 patients who received FIRAZYR 30 mg (n=113), placebo (n=75), or comparator ...
-
7 DRUG INTERACTIONS7.1 ACE Inhibitors - FIRAZYR is a bradykinin B2 receptor antagonist and thereby has the potential to have a pharmacodynamic interaction with ACE inhibitors where FIRAZYR may attenuate the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and the pharmacovigilance database with Firazyr (icatibant) use in pregnant women have not identified a drug-associated ...
-
10 OVERDOSAGEIn a clinical study evaluating a 90 mg dose (30 mg in each of 3 subcutaneous sites), the adverse event profile was similar to that seen with 30 mg administered in a single subcutaneous site. In ...
-
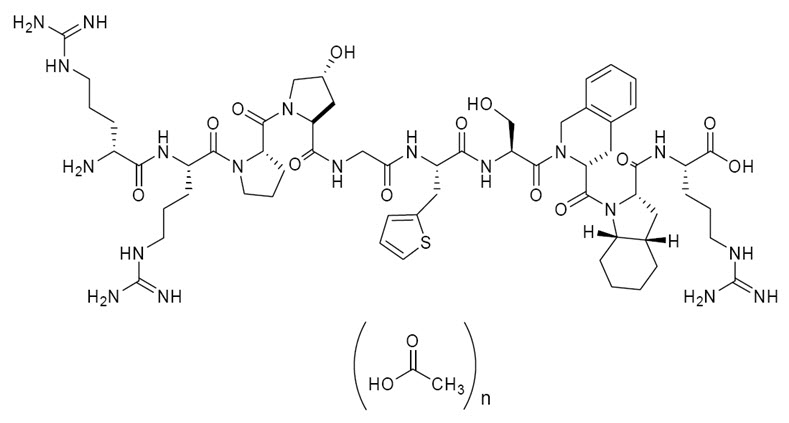
11 DESCRIPTIONFIRAZYR (icatibant) is a synthetic decapeptide with five non-proteinogenic amino acids. The chemical structure of icatibant acetate is presented in Figure 1. Figure 1 Chemical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Icatibant is a competitive antagonist selective for the bradykinin B2 receptor, with an affinity similar to bradykinin. Hereditary angioedema is caused by an absence ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year studies were conducted in CD1 mice and Wistar rats to assess the carcinogenic potential of FIRAZYR. No evidence of ...
-
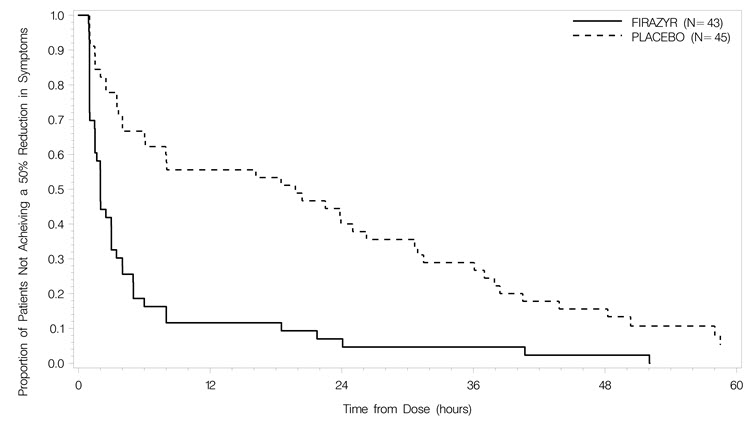
14 CLINICAL STUDIESThe efficacy and safety of FIRAZYR for the treatment of acute attacks of HAE in adults were studied in three controlled clinical trials. Among the 223 patients in these studies, the mean age was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - FIRAZYR is supplied as a single-dose, prefilled syringe for subcutaneous administration. Each syringe delivers 3 mL of a sterile solution of icatibant 30 mg (as icatibant ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). 17.1 Information for Patients - Patients may self-administer FIRAZYR upon recognition ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Takeda Pharmaceuticals America, Inc. Lexington, MA 02421 - FIRAZYR® and the FIRAZYR Logo® are registered trademarks of Shire Orphan Therapies GmbH. TAKEDA® and the TAKEDA Logo® are ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - FIRAZYR (FIR-a-zeer) (icatibant) Injection, for subcutaneous use - Please read this Patient Information before you use FIRAZYR and each time you get a refill. There may ...
-
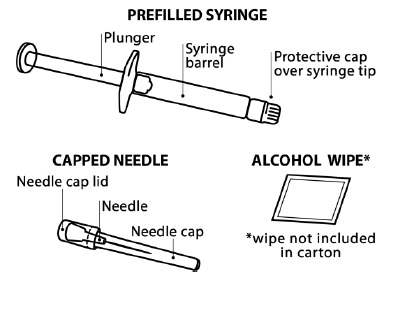
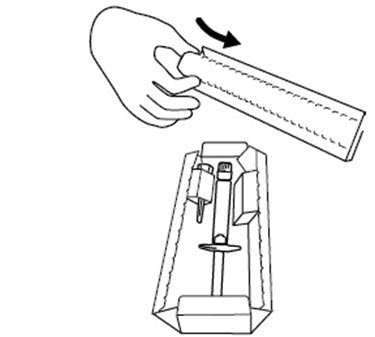
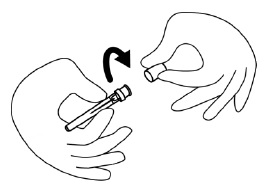
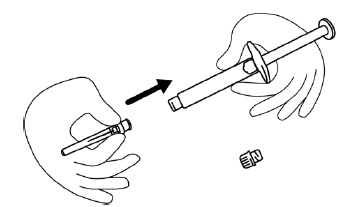
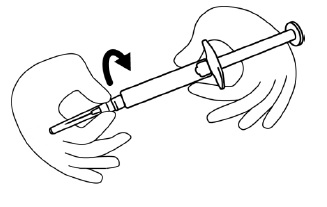
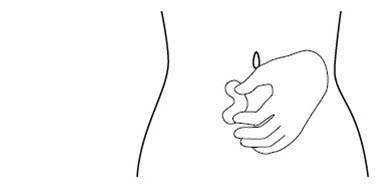
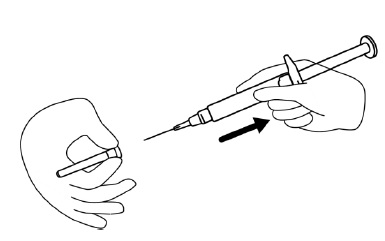
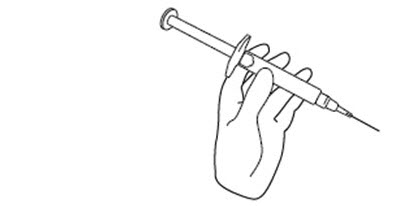
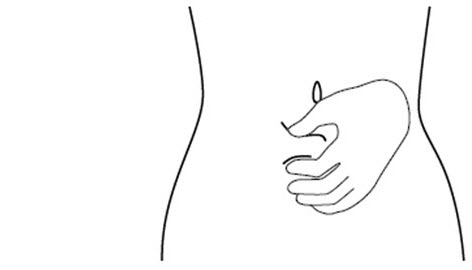
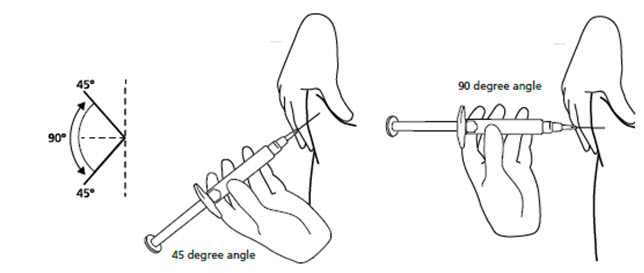
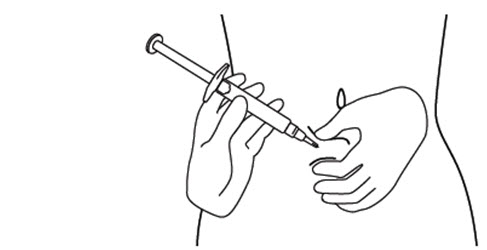
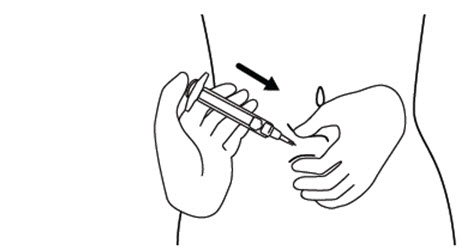
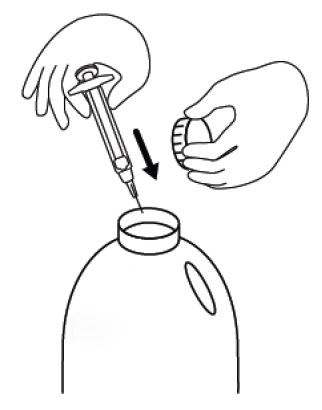
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - FIRAZYR (FIR-a-zeer) (icatibant) Injection, for subcutaneous use - Step 1. Preparing your dose of FIRAZYR - Wash your hands with soap and water. You will need the ...
-
PRINCIPAL DISPLAY PANEL - 3 mL Syringe Carton - 54092-702-02Takeda - Prefilled Syringe - Rx ONLY - NDC 54092-702-02 - Carton contains: One single-dose, prefilled syringe and one 25G - hypodermic needle. Full prescribing information with - patient injection ...
-
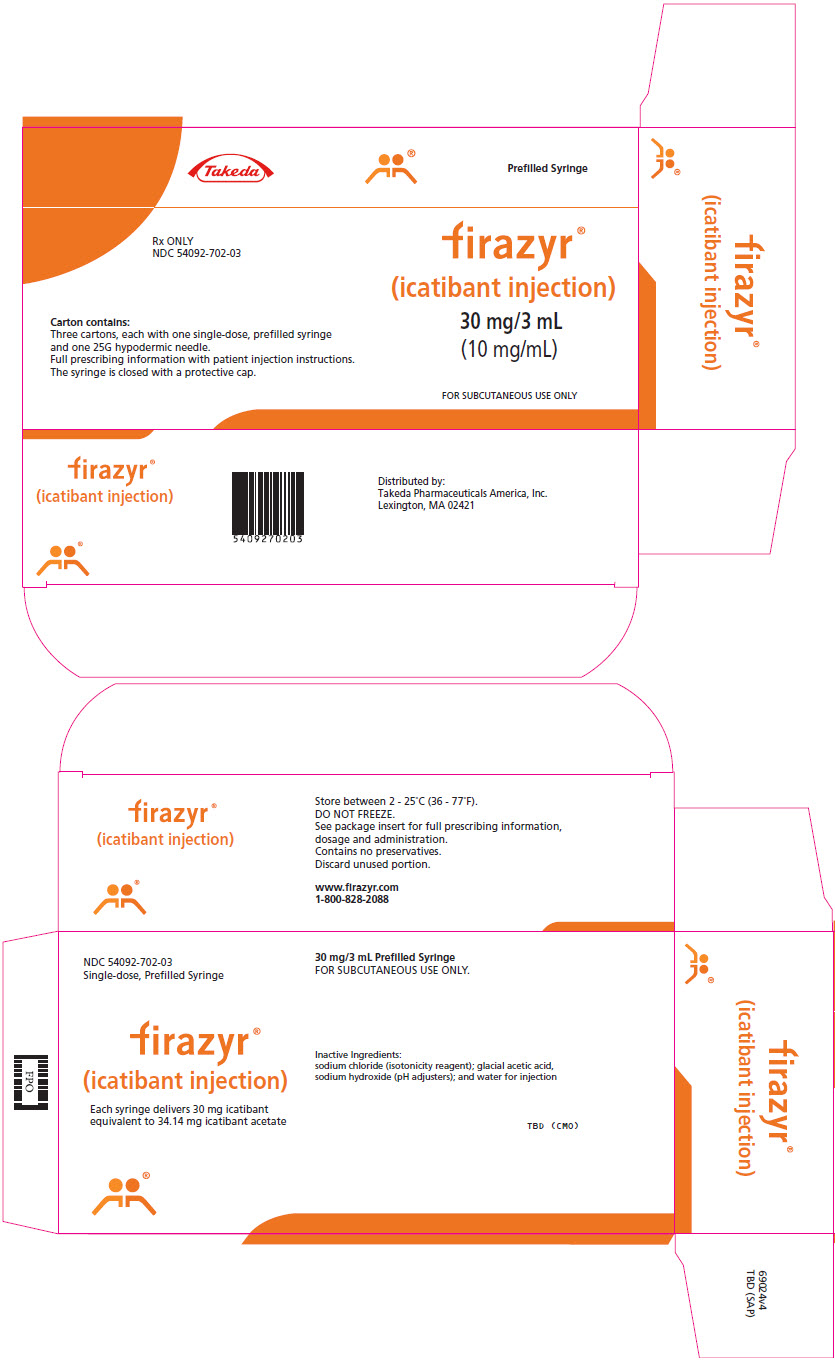
PRINCIPAL DISPLAY PANEL - 3 mL Syringe Carton - 54092-702-03Takeda - Prefilled Syringe - Rx ONLY - NDC 54092-702-03 - Carton contains: Three cartons, each with one single-dose, prefilled syringe - and one 25G hypodermic needle. Full prescribing information with ...
-
PRINCIPAL DISPLAY PANEL - 3 mL Syringe Carton - 54092-702-01Takeda - Prefilled Syringe - Rx ONLY - NDC 54092-702-01 - Sample - Not For Sale - Carton contains: One single-dose, prefilled syringe and one 25G - hypodermic needle. Full prescribing information with ...
-
INGREDIENTS AND APPEARANCEProduct Information