Label: FIASP- insulin aspart injection injection, solution
FIASP- insulin aspart injection, solution
-
NDC Code(s):
0169-3201-11,
0169-3201-90,
0169-3204-15,
0169-3204-90, view more0169-3204-97, 0169-3205-11, 0169-3205-15, 0169-3205-91, 0169-3205-95, 0169-3206-11, 0169-3206-15, 0169-3206-91, 0169-3206-95
- Packager: Novo Nordisk
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FIASP safely and effectively. See full prescribing information for FIASP. FIASP® (insulin aspart) injection, for subcutaneous or ...These highlights do not include all the information needed to use FIASP safely and effectively. See full prescribing information for FIASP.
FIASP® (insulin aspart) injection, for subcutaneous or intravenous use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
- •
- FIASP is a rapid-acting human insulin analog indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus (1).
DOSAGE AND ADMINISTRATION
- •
- Individualize and adjust the dosage of FIASP based on route of administration, individual’s metabolic needs, blood glucose monitoring results and glycemic control goal (2.3).
- •
- Dosage adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns, changes in renal or hepatic function or during acute illness (2.3).
- •
-
Subcutaneous injection (2.2):
- o
- Inject at the start of a meal or within 20 minutes after starting a meal into the abdomen, upper arm, or thigh.
- o
- Rotate injection sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis.
- o
- Should generally be used in regimens with an intermediate- or long-acting insulin.
- •
-
Continuous Subcutaneous Infusion (Insulin Pump) (2.2):
- o
- Refer to the insulin infusion pump user manual to see if FIASP can be used. Use in accordance with the insulin pumps’ instructions for use.
- o
- Administer by continuous subcutaneous infusion using an insulin pump in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis.
- •
- Intravenous Infusion: Administer only under medical supervision after diluting to concentrations from 0.5 to 1 unit/mL insulin aspart in infusion systems using polypropylene infusion bags (2.2).
DOSAGE FORMS AND STRENGTHS
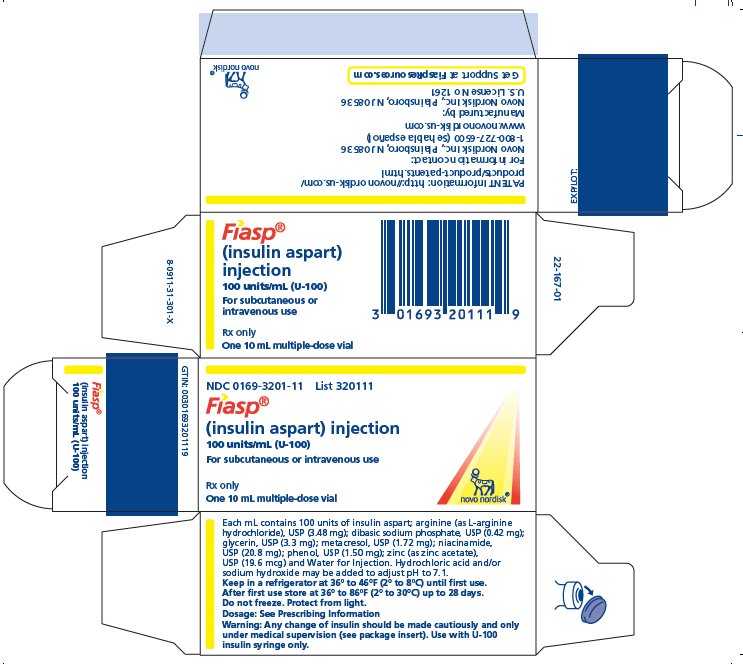
Injection: 100 units/mL (U-100) available as:
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Never share a FIASP FlexTouch pen, PenFill cartridge or PenFill cartridge device between patients, even if the needle is changed (5.1).
- •
- Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient’s insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring (5.2).
- •
- Hypoglycemia: May be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness (5.3).
- •
- Hypoglycemia due to medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection (5.4).
- •
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated (5.5).
- •
- Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue FIASP, monitor and treat if indicated (5.6).
- •
- Fluid retention and heart failure with concomitant use of thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs (5.7).
- •
- Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Monitor glucose and administer FIASP by subcutaneous injection if pump malfunction occurs (5.8).
ADVERSE REACTIONS
Adverse reactions observed with FIASP include: hypoglycemia, allergic reactions, hypersensitivity, injection/infusion site reactions, lipodystrophy, and weight gain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-800-727-6500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Drugs that Increase Hypoglycemia Risk or Increase or Decrease Blood Glucose Lowering Effect: Adjustment of dosage may be needed; closely monitor blood glucose (7).
- •
- Drugs that Blunt Hypoglycemia Signs and Symptoms (e.g., beta-blockers, clonidine, guanethidine, and reserpine): Increased frequency of glucose monitoring may be required (7).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Route of Administration Instructions
2.3 Dosage Recommendations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a FIASP FlexTouch Pen, PenFill Cartridge or PenFill Cartridge Device Between Patients
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
5.3 Hypoglycemia
5.4 Hypoglycemia Due to Medication Errors
5.5 Hypokalemia
5.6 Hypersensitivity and Allergic Reactions
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-Gamma Agonists
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
14.2 Type 1 Diabetes Mellitus - Adults
14.3 Type 1 Diabetes Mellitus - Pediatric Patients
14.4 Type 2 Diabetes Mellitus - Adults
14.5 Type 1 Diabetes Mellitus– Adult Continuous Subcutaneous Insulin Infusion (CSII)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Recommended Storage
17 PATIENT COUNSELING INFORMATION
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE FIASP is indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus.
FIASP is indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus.
Close -
2 DOSAGE AND ADMINISTRATION 2.1 Important Preparation and Administration Instructions - • Always check insulin label before administration [see Warnings and Precautions (5.4)]. • Inspect FIASP visually before use. It ...
2.1 Important Preparation and Administration Instructions
- •
- Always check insulin label before administration [see Warnings and Precautions (5.4)].
- •
- Inspect FIASP visually before use. It should appear clear and colorless. Do not use FIASP if particulate matter or coloration is seen.
- •
- Use FIASP FlexTouch pen with caution in patients with visual impairment who may rely on audible clicks to dial their dose.
- •
- Use PenFill cartridges with caution in patients with visual impairment.
- •
- Do not mix FIASP with any other insulin.
2.2 Route of Administration Instructions
Subcutaneous Injection:
- •
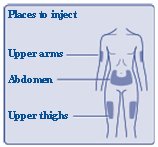
- Inject FIASP at the start of a meal or within 20 minutes after starting a meal subcutaneously into the abdomen, upper arm, or thigh.
- •
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Adverse Reactions (6.1, 6.2)].
- •
- FIASP given by subcutaneous injection should generally be used in regimens with intermediate or long-acting insulin.
- •
- Instruct patients on basal-bolus treatment who forget a mealtime dose to monitor their blood glucose level to decide if an insulin dose is needed, and to resume their usual dosing schedule at the next meal.
- •
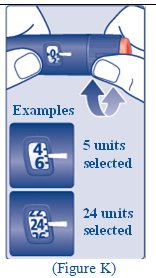
- The FIASP FlexTouch pen dials in 1 unit increments.
Continuous Subcutaneous Infusion (Insulin Pump):
- •
- Refer to the continuous subcutaneous insulin infusion pump user manual to see if FIASP or FIASP PumpCart can be used with the insulin pump. Use FIASP and FIASP PumpCart in accordance with the insulin pump system’s instructions for use.
- •
- Administer FIASP by continuous subcutaneous infusion in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not infuse into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) Adverse Reactions (6.1)].
- •
- Train patients using continuous subcutaneous insulin infusion therapy to administer insulin by injection and have alternate insulin therapy available in case of insulin pump failure [see Warnings and Precautions (5.8)].
- •
- Change FIASP in the pump reservoir at least every 6 days or replace the PumpCart cartridge at least every 4 days, or according to the pump user manual, whichever is shorter. Follow the FIASP-specific information for in-use time because FIASP-specific information may differ from general insulin pump user manual instructions.
- •
- Change the infusion sets and the infusion set insertion site according to the manufacturers user manual.
- •
- Do not mix with other insulins or diluents in the insulin pump.
- •
- Do not expose FIASP in the pump reservoir to temperatures greater than 37°C (98.6°F).
Intravenous Administration:
- •
- Administer FIASP intravenously only under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)].
- •
- Dilute FIASP to concentrations from 0.5 unit/mL to 1 unit/mL insulin aspart in infusion systems using polypropylene infusion bags.
- •
- FIASP is stable at room temperature at 20°C to 25°C (68°F to 77°F) for 24 hours in 0.9% sodium chloride or 5% dextrose infusion fluids.
Close2.3 Dosage Recommendations
- •
- Individualize the dosage of FIASP based on the route of administration, patient’s metabolic needs, blood glucose monitoring results, and glycemic control goal.
- •
- If converting from another mealtime insulin to FIASP, the initial change can be done on a unit-to-unit basis.
- •
- Dose adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycemia or hyperglycemia [see Warnings and Precautions (5.2, 5.3) and Drug Interactions (7), and Use in Specific Populations (8.6, 8.7)].
- •
- Closely monitor blood glucose when converting insulins used in insulin pumps as individualization of insulin pump parameters may be necessary to minimize the risk of hypoglycemia and hyperglycemia [see Warnings and Precautions (5.2, 5.3) and Adverse Reactions (6.1)]
- •
- During changes to a patient’s insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- •
- Dosage adjustment may be needed when FIASP is co-administered with certain drugs [see Drug Interactions (7)].
-
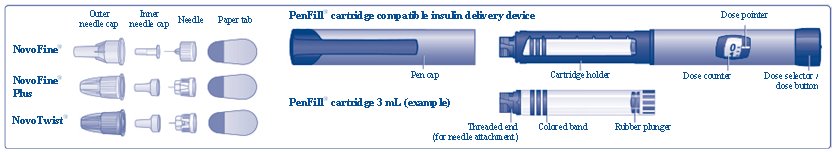
3 DOSAGE FORMS AND STRENGTHS Injection: 100 units of insulin aspart per mL (U-100) is available as a clear and colorless solution in: • 10 mL multiple-dose vial - • 3 mL single-patient-use FIASP FlexTouch pen - • 3 mL ...
Injection: 100 units of insulin aspart per mL (U-100) is available as a clear and colorless solution in:
- •
- 10 mL multiple-dose vial
- •
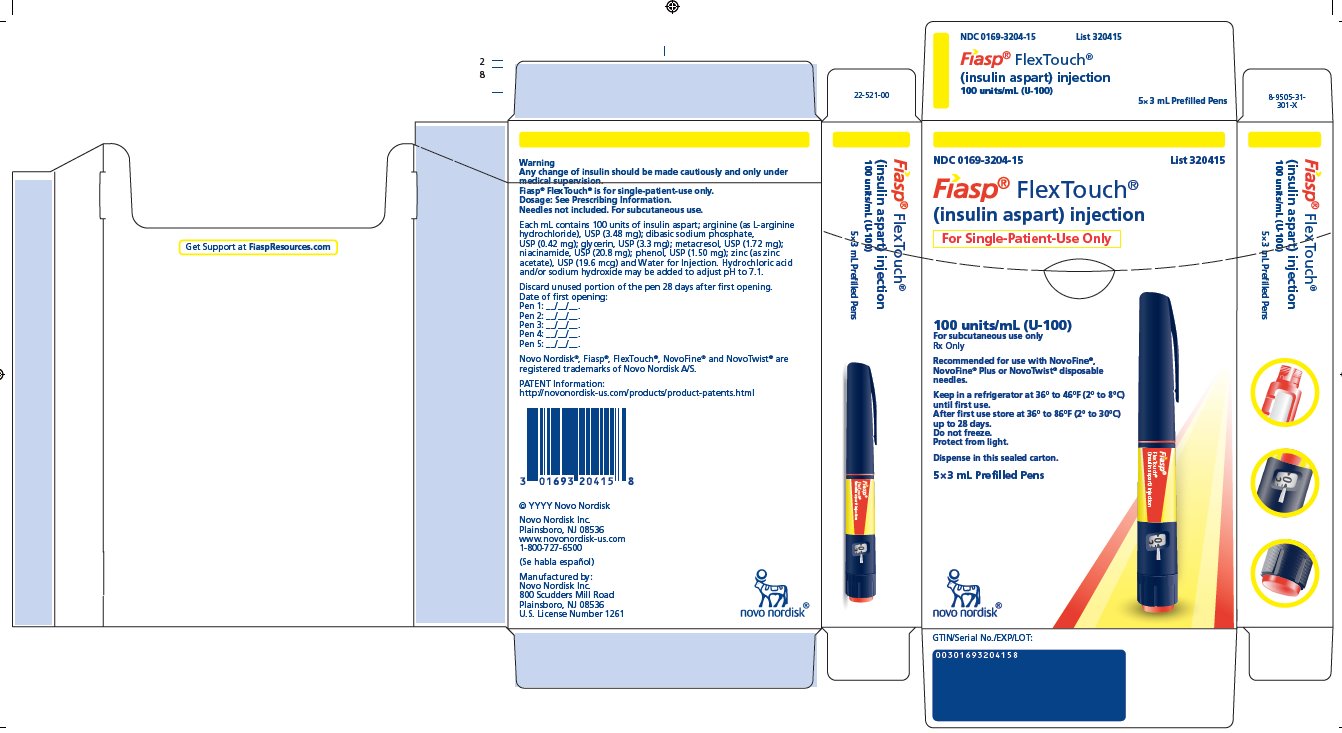
- 3 mL single-patient-use FIASP FlexTouch pen
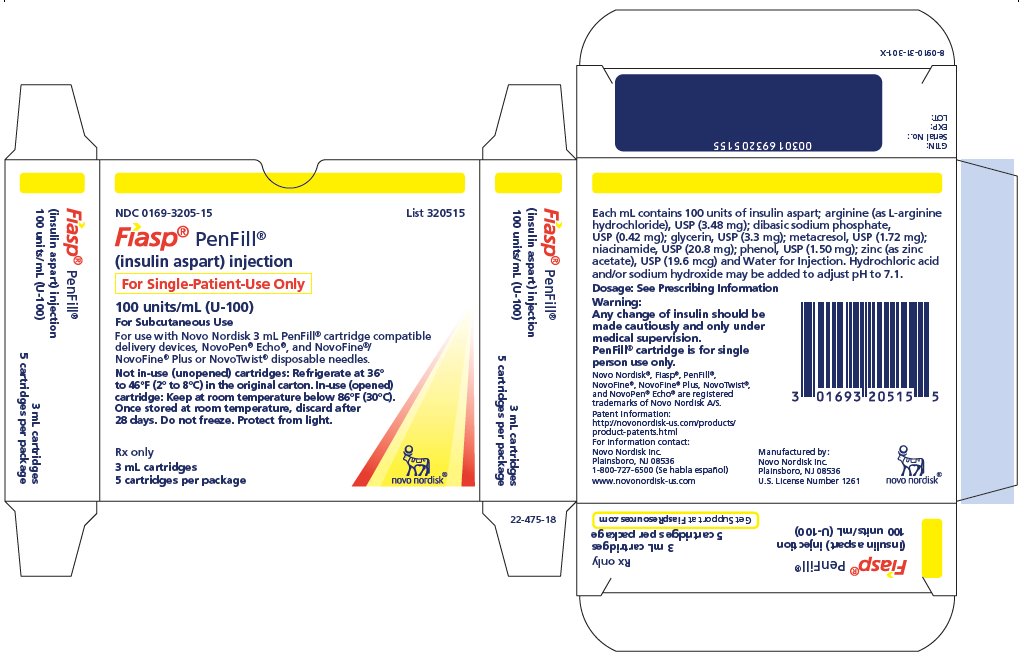
- •
- 3 mL single-patient-use PenFill cartridges for use in a PenFill cartridge delivery device
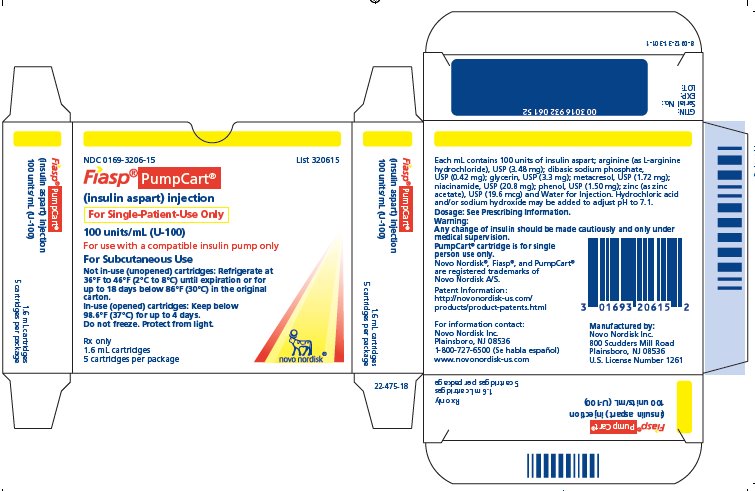
- •
- 1.6 mL single-patient-use PumpCart cartridges for use in a compatible insulin pump.
-
4 CONTRAINDICATIONS FIASP is contraindicated: • During episodes of hypoglycemia [see Warnings and Precautions (5.3)]. • In patients with known hypersensitivity to insulin aspart or any of the excipients in FIASP ...
FIASP is contraindicated:
- •
- During episodes of hypoglycemia [see Warnings and Precautions (5.3)].
- •
- In patients with known hypersensitivity to insulin aspart or any of the excipients in FIASP [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Never Share a FIASP FlexTouch Pen, PenFill Cartridge or PenFill Cartridge Device Between Patients - FIASP FlexTouch disposable pen, PenFill cartridge and PenFill cartridge devices should ...
5.1 Never Share a FIASP FlexTouch Pen, PenFill Cartridge or PenFill Cartridge Device Between Patients
FIASP FlexTouch disposable pen, PenFill cartridge and PenFill cartridge devices should never be shared between patients, even if the needle is changed. Patients using FIASP vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6.1, 6.2)].
Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes mellitus, dosage adjustments in concomitant anti-diabetic treatment may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulin therapies, including FIASP [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). FIASP, or any insulin, should not be used during episodes of hypoglycemia [see Contraindications (4)].
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. As with all insulin preparations, the glucose lowering effect time course of FIASP may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Use in Specific Populations (8.4), Clinical Pharmacology (12.2)].
Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [seeDrug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [seeUse in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between FIASP and other insulins, instruct patients to always check the insulin label before each injection.
5.5 Hypokalemia
All insulin products, including FIASP, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to potassium concentrations).
5.6 Hypersensitivity and Allergic Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including FIASP [see Adverse Reactions (6.1)]. If hypersensitivity reactions occur, discontinue FIASP; treat per standard of care and monitor until symptoms and signs resolve. FIASP is contraindicated in patients who have had hypersensitivity reactions to insulin aspart, or any of the excipients in FIASP [see Contraindications (4)].
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-Gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including FIASP, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
Close5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of FIASP may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16.2), and Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in labeling: • Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen [see Warnings and Precautions ...
The following clinically significant adverse reactions are described elsewhere in labeling:
- •
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen [see Warnings and Precautions (5.2)]
- •
- Hypoglycemia [see Warnings and Precautions (5.3)]
- •
- Hypokalemia [see Warnings and Precautions (5.5)]
- •
- Hypersensitivity and allergic reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates actually observed in clinical practice.
The data in Table 1 reflect the exposure of 763 adult patients with type 1 diabetes mellitus to FIASP in one clinical trial with a mean exposure duration of 25 weeks [see Clinical Studies (14.2)]. The mean age was 44.4 years and the mean duration of diabetes was 19.9 years. 59% were male, 93% were White, and 2% were Black or African American; and 7% were Hispanic or Latino. The mean BMI was 26.7 kg/m2 and the mean HbA1c at baseline was 7.6%.
The data in Table 2 reflect the exposure of 341 adult patients with type 2 diabetes mellitus to FIASP in one clinical trial with a mean exposure duration of 24 weeks [see Clinical Studies (14)]. The mean age was 59.6 years and the mean duration of diabetes was 13.2 years. 47% were male, 80% were White, and 6% were Black or African American; and 8% were Hispanic or Latino. The mean BMI was 31.5 kg/m2 and the mean HbA1c at baseline was 8.0%.
The data in Table 3 reflect the exposure of 519 pediatric patients with type 1 diabetes mellitus to FIASP in one clinical trial with a mean exposure duration of 26 weeks [see Clinical Studies (14.3)]. The mean age was 11.7 years and the mean duration of diabetes mellitus was 4.4 years. 54% were male, 81% were White, 16% were Asian and 2% were Black or African American. The mean BMI was 19.7 kg/m2 and the mean HbA1c at baseline was 7.6%.
Common adverse reactions, excluding hypoglycemia, were defined as events occurring in ≥5% and occurring at the same rate or greater for FIASP-treated patients than comparator-treated patients.
Table 1. Adverse Reactions (%*) in Adult Patients with Type 1 Diabetes Mellitus
Mealtime FIASP + Insulin detemir
(N=386)Postmeal FIASP + Insulin detemir
(N=377)Nasopharyngitis
20.2
23.9
Upper respiratory tract infection
9.1
7.4
Nausea
4.9
5.0
Diarrhea
5.4
3.2
Back pain
5.2
4.0
*Incidence ≥ 5% and occurring at the same rate or greater with FIASP than comparator
Table 2. Adverse Reactions (%*) in Adult Patients with Type 2 Diabetes Mellitus
FIASP + Insulin glargine
(N=341)Urinary tract infection
5.9
*Incidence ≥ 5% and occurring at the same rate or greater with FIASP than comparator
Table 3: Adverse Reactions (%*) in Pediatric Patients with Type 1 Diabetes Mellitus
Mealtime FIASP + Insulin degludec
(N=261)
Postmeal FIASP + Insulin degludec
(N=258)
Viral upper respiratory tract infection
Upper respiratory tract infection
Influenza
Rhinitis
Headache
Pyrexia
Vomiting
23.0
8.4
7.7
3.8
6.1
8.4
3.4
20.5
12.4
5.8
6.2
10.1
6.2
8.1
*Incidence ≥ 5% and occurring at the same rate or greater with FIASP than comparator
Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including FIASP. The rates of reported hypoglycemia depend on the definition of hypoglycemia used, diabetes type, insulin dose, intensity of glucose control, background therapies, and other intrinsic and extrinsic patient factors. For these reasons, comparing rates of hypoglycemia in clinical trials for FIASP with the incidence of hypoglycemia for other products may be misleading and also, may not be representative of hypoglycemia rates that occur in clinical practice.
Incidence rates for severe hypoglycemia in adults with type 1 and type 2 diabetes mellitus and pediatric patients with type 1 diabetes mellitus treated with FIASP in clinical trials are shown in Table 4 [see Clinical Studies (14)].
Table 4. Proportion (%) of Patients with Type 1 Diabetes and Type 2 Diabetes Mellitus Experiencing at Least One Episode of Severe Hypoglycemia in Adult and Pediatric Clinical Trials
Study A (Type 1)
Adults
Study B (Type 2)
Adults
Study E (Type 1)
Pediatric
Study D
(Type 1 CSII)
Mealtime FIASP + Insulin detemir
(N=386)
Postmeal FIASP + Insulin detemir
(N=377)
FIASP + Insulin glargine
(N=341)
Mealtime FIASP + Insulin degludec
(N=261)
Postmeal FIASP + Insulin degludec
(N=258)
FIASP
(N=236)
Severe hypoglycemia*
6.7
8.0
3.2
1.1
3.1
4.7
- *Severe hypoglycemia: an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions
Blood glucose confirmed hypoglycemia was defined as a self-measured glucose calibrated to plasma of less than 56 mg/dL.
In Study D, adult patients with type 1 diabetes mellitus treated with FIASP in a pump reported a higher rate of blood glucose confirmed hypoglycemic episodes within the first hour after a meal compared to patients treated with NovoLog [see Clinical Trials (14.5)].
In Study E, pediatric patients with type 1 diabetes mellitus treated with mealtime and postmeal FIASP reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog; the imbalance was greater during the nocturnal period [see Use in Specific Populations (8.4), Clinical Trials (14.3)].
Allergic Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, angioedema, bronchospasm, hypotension, and shock may occur with any insulin, including FIASP, and may be life threatening. In the clinical program, generalized hypersensitivity reactions (manifested by generalized skin rash and facial edema) were reported in 0.4% of adult patients treated with FIASP. Allergic skin manifestations reported with FIASP in 1.7% of adult patients from the clinical program include eczema, rash, rash pruritic, urticaria and dermatitis. In Study D, allergic reactions were reported in 4.2% of adult patients with type 1 diabetes mellitus treated with FIASP. In Study E, allergic reactions were reported in 4% of pediatric patients with type 1 diabetes mellitus treated with FIASP.
Lipodystrophy
Administration of insulin, including FIASP, has resulted in lipohypertrophy (enlargement or thickening of tissue) and lipoatrophy (depression in the skin). In the clinical program, lipodystrophy was reported in 0.4% of adult patients and 2.1% of pediatric patients treated with FIASP [see Dosage and Administration (2.2)].
Injection/Infusion Site Reactions
As with other insulin therapy, patients may experience rash, redness, inflammation, pain, bruising or itching at the site of FIASP injection or infusion. These reactions usually resolve in a few days to a few weeks, but in some occasions, may require discontinuation of FIASP. In the clinical program, injection site reactions occurred in 1.6% of adult patients treated with FIASP. In Study A, adult patients with type 1 diabetes mellitus treated with FIASP reported 2.2% injection site reactions. In Study D, infusion site reactions were reported in 10.2% of adult patients with type 1 diabetes mellitus treated with FIASP. In Study E, injection site reactions were reported in 4.2% of pediatric patients with type 1 diabetes mellitus treated with FIASP.
Weight Gain
Weight gain can occur with insulin therapy, including FIASP, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria. In Study A, adult patients with type 1 diabetes mellitus treated with FIASP gained an average of 0.7 kg and in Study B, adult patients with type 2 diabetes mellitus treated with FIASP gained an average of 2.7 kg.
Peripheral Edema
Insulin, including FIASP, may cause sodium retention and edema, particularly if previous poor metabolic control is improved by intensified insulin therapy. In the clinical program, peripheral edema occurred in 0.8% of adult patients treated with FIASP.
Close6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of insulin aspart. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
-
7 DRUG INTERACTIONS Table 5 includes clinically significant drug interactions with FIASP. Table 5. Clinically Significant Drug Interactions with FIASP - Drugs That May Increase the Risk of ...
Table 5 includes clinically significant drug interactions with FIASP.
Table 5. Clinically Significant Drug Interactions with FIASP
CloseDrugs That May Increase the Risk of Hypoglycemia
Drugs:
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics.
Intervention:
Dose reductions and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs.
Drugs That May Decrease the Blood Glucose Lowering Effect of FIASP
Drugs:
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
Intervention:
Dose increases and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs.
Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of FIASP
Drugs:
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
Intervention:
Dose adjustment and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs.
Drugs That May Blunt Signs and Symptoms of Hypoglycemia
Drugs:
Beta-blockers, clonidine, guanethidine, and reserpine.
Intervention:
Increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available data with FIASP in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. Available information from ...
8.1 Pregnancy
Risk Summary
There are no available data with FIASP in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. Available information from published randomized controlled trials with insulin aspart use during the second trimester of pregnancy have not reported an association with insulin aspart and major birth defects or adverse maternal or fetal outcomes [see Data]. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
In animal reproduction studies, administration of subcutaneous insulin aspart to pregnant rats and rabbits during the period of organogenesis did not cause adverse developmental effects at exposures 8- times and equal to the human subcutaneous dose of 1.0 unit/kg/day, respectively. Pre- and post-implantation losses and visceral/skeletal abnormalities were seen at higher exposures, which are considered secondary to maternal hypoglycemia. These effects were similar to those observed in rats administered regular human insulin [see Data].
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7% and has been reported to be as high as 20-25% in women with a HbA1c >10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from 5 randomized controlled trials of 441 pregnant women with diabetes mellitus treated with insulin aspart starting during the late 2nd trimester of pregnancy did not identify an association of insulin aspart with major birth defects or adverse maternal or fetal outcomes. However, these studies cannot definitely establish the absence of any risk because of methodological limitations, including a variable duration of treatment and small size of the majority of the trials.
Animal Data
Fertility, embryo-fetal and pre-and postnatal development studies have been performed with insulin aspart and regular human insulin in rats and rabbits. In a combined fertility and embryo-fetal development study in rats, insulin aspart was administered before mating, during mating, and throughout pregnancy. Further, in a pre- and postnatal development study insulin aspart was given throughout pregnancy and during lactation to rats. In an embryo-fetal development study insulin aspart was given to female rabbits during organogenesis. The effects of insulin aspart did not differ from those observed with subcutaneous regular human insulin. Insulin aspart, like human insulin, caused pre- and post-implantation losses and visceral/skeletal abnormalities in rats at a dose of 200 units/kg/day (approximately 32 times the human subcutaneous dose of 1.0 unit/kg/day, based on human exposure equivalents) and in rabbits at a dose of 10 units/kg/day (approximately three times the human subcutaneous dose of 1.0 unit/kg/day, based on human exposure equivalents). No significant effects were observed in rats at a dose of 50 units/kg/day and in rabbits at a dose of 3 units/kg/day. These doses are approximately 8 times the human subcutaneous dose of 1.0 unit/kg/day for rats and equal to the human subcutaneous dose of 1.0 unit/kg/day for rabbits, based on human exposure equivalents. The effects are considered secondary to maternal hypoglycemia.
8.2 Lactation
Risk Summary
There are no data on the presence of FIASP in human milk, the effects on the breastfed infant, or the effect on milk production. One small published study reported that exogenous insulin, including insulin aspart, was present in human milk. However, there is insufficient information to determine the effects of insulin aspart on the breastfed infant and no available information on the effects of insulin aspart on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for insulin, any potential adverse effects on the breastfed child from FIASP or insulin aspart or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of FIASP have been established to improve glycemic control in pediatric patients with diabetes mellitus. Use of FIASP for this indication is supported by evidence from an adequate and well-controlled study in 777 pediatric patients with type 1 diabetes mellitus aged 2 to 17 years, and from studies in adults with diabetes mellitus [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
Pediatric patients with type 1 diabetes mellitus treated with mealtime and postmeal FIASP reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients [see Warnings and Precautions (5.3), Clinical Trial Experience (6.1)].
8.5 Geriatric Use
In the three controlled clinical studies, 192 of 1219 (16%) FIASP treated patients with type 1 or type 2 diabetes mellitus were ≥ 65 years of age and 24 of 1219 (2%) were ≥ 75 years of age. No overall differences in safety or effectiveness were observed between these elderly patients and younger adult patients.
Nevertheless, caution should be exercised when FIASP is administered to geriatric patients. In elderly patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemia [see Warnings and Precautions (5.3), Adverse Reactions (6.1) and Clinical Studies (14)].
Pharmacokinetic/pharmacodynamic study to assess the effect of age on the onset of FIASP action has been performed [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Patients with renal impairment may be at increased risk of hypoglycemia and may require more frequent FIASP dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
Patients with hepatic impairment may be at increased risk of hypoglycemia and may require more frequent FIASP dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)]. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments ...
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)]. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed. More severe episodes with coma, seizure or neurologic impairment may be treated with an intramuscular/subcutaneous glucagon product for emergency use or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately.
Close -
11 DESCRIPTION Insulin aspart is a rapid-acting insulin analog for subcutaneous or intravenous administration. Insulin aspart is homologous with regular human insulin with the exception of a single substitution ...
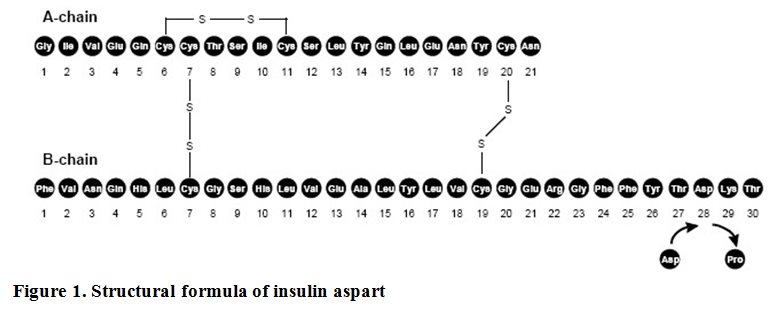
Insulin aspart is a rapid-acting insulin analog for subcutaneous or intravenous administration. Insulin aspart is homologous with regular human insulin with the exception of a single substitution of the amino acid proline by aspartic acid in position B28, and is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae. Insulin aspart has the empirical formula C256H381N65079S6 and a molecular weight of 5825.8 daltons.
FIASP (insulin aspart) injection is an aqueous, sterile, clear and colorless solution. Each mL contains 100 units of insulin aspart and the inactive ingredients: arginine (as L-arginine hydrochloride), USP (3.48 mg); dibasic sodium phosphate, USP (0.42 mg); glycerin, USP (3.3 mg); metacresol, USP (1.72 mg); niacinamide, USP (20.8 mg); phenol, USP (1.50 mg); zinc (as zinc acetate), USP (19.6 mcg) and Water for Injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH to 7.1.
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - The primary activity of FIASP is the regulation of glucose metabolism. Insulins, including insulin aspart, the active ingredient in FIASP, exert their specific action ...
12.1 Mechanism of Action
The primary activity of FIASP is the regulation of glucose metabolism. Insulins, including insulin aspart, the active ingredient in FIASP, exert their specific action through binding to insulin receptors. Receptor-bound insulin lowers blood glucose by facilitating cellular uptake of glucose into skeletal muscle and adipose tissue and by inhibiting the output of glucose from the liver. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
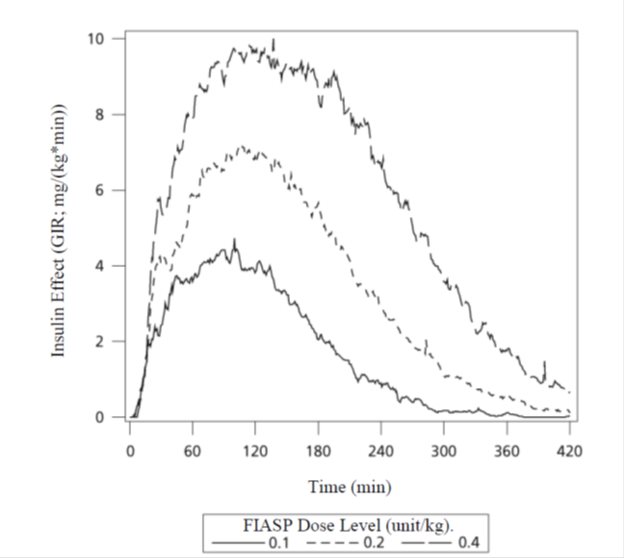
The time course of insulin action (i.e., glucose lowering) may vary considerably in different individuals or within the same individual. The average pharmacodynamic profile (i.e., glucose lowering effect measured as glucose infusion rate (GIR) in a euglycemic clamp study) for subcutaneous administration of 0.1, 0.2, and 0.4 unit/kg of FIASP in 46 patients with Type 1 diabetes mellitus is shown in Figure 2 and key characteristics of the timing of the effect are described in Table 6 below.
Table 6. Timing of insulin effect (i.e., mean pharmacodynamic effect) after subcutaneous administration of 0.1, 0.2 and 0.4 unit/kg of FIASP in patients (N=46) with Type 1 Diabetes Mellitus and corresponding to the data shown in Figure 2
Parameter for Insulin Effect
FIASP
0.1 unit/kg
FIASP
0.2 unit/kg
FIASP
0.4 unit/kg
Time to first measurable effect
~20 minutes
~17 minutes
~16 minutes
Time to peak effect
~91 minutes
~122 minutes
~133 minutes
Time for effect to return to baseline
~5 hours
~6 hours
~7 hours
Figure 2. Mean insulin effect (i.e., mean pharmacodynamic effect) over time after subcutaneous administration of 0.1, 0.2 and 0.4 unit/kg of FIASP in patients (N=46) with Type 1 Diabetes Mellitus
On average, the pharmacodynamic effects of FIASP, measured as area under the glucose infusion rate-time curve (AUCGIR), was 697 mg/kg, 1406 mg/kg, and 2427 mg/kg following administration of 0.1, 0.2, and 0.4 unit/kg of FIASP.
The day-to-day variability in glucose-lowering-effect of FIASP within patients was ~18% for total glucose lowering (AUCGIR, 0-12h) and ~19% for maximum glucose lowering effect (GIRmax).
12.3 Pharmacokinetics
Absorption
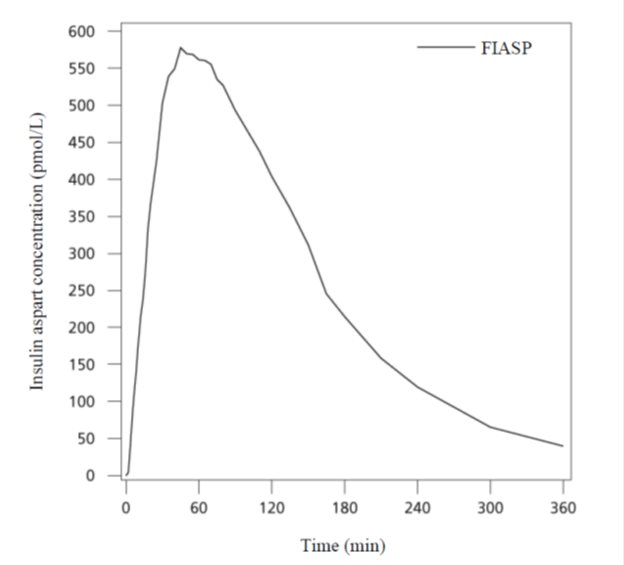
Pharmacokinetic results from a euglycemic clamp study in adult patients with type 1 diabetes mellitus (N=51) showed that insulin aspart appeared in the circulation ~2.5 minutes after administration of FIASP (Figure 3). Time to maximum insulin concentrations was achieved ~63 minutes after administration of FIASP.
Figure 3. Mean Insulin Aspart Serum Concentration Profile in Adult Subjects with Type 1 Diabetes Mellitus (N=51) following a single 0.2 unit/kg dose (subcutaneous) of FIASP
Total insulin exposure and maximum insulin concentration increase proportionally with increasing subcutaneous dose of FIASP within the therapeutic dose range.
Distribution
Insulin aspart has a low binding affinity to plasma proteins (<10%), similar to that seen with regular human insulin.
Elimination
The apparent terminal half-life after subcutaneous administration of FIASP is about 1.1 hours.
Specific Populations
Age, gender, BMI, and race did not meaningfully affect the pharmacokinetics and pharmacodynamics of FIASP.
Patients with Renal and Hepatic Impairment
Based on studies conducted with insulin aspart, renal and hepatic impairment is not known to impact the pharmacokinetics of insulin aspart.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of FIASP or of other insulin aspart products.
In a 26-week study in adult subjects with type 1 diabetes mellitus (Study A) [see Clinical Studies (14.2)], among the 763 subjects who received FIASP, 97% were positive for cross-reacting anti-insulin antibodies (AIA) at least once during the study, including 90% that were positive at baseline. A total of 25% of patients who received FIASP were positive for anti-drug (insulin aspart) antibodies (ADA) at least once during the study, including 17% that were positive at baseline.
In a 26-week study in pediatric subjects with type 1 diabetes mellitus (Study E) [see Clinical Studies (14.3)], among the 519 subjects who received FIASP, 97% were positive for cross-reacting AIA at least once during the study, including 95% that were positive at baseline. A total of 19% of patients who received FIASP were positive for ADA at least once during the study, including 16.0% that were positive at baseline.
There was no identified clinically significant effect of ADA on pharmacokinetics, pharmacodynamics, safety, or effectiveness of FIASP.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In 52-week studies, Sprague-Dawley rats were dosed subcutaneously with insulin aspart at 10, 50, and 200 units/kg/day (approximately ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 52-week studies, Sprague-Dawley rats were dosed subcutaneously with insulin aspart at 10, 50, and 200 units/kg/day (approximately 2, 8, and 32 times the human subcutaneous dose of 1.0 units/kg/day, based on units/body surface area, respectively). At a dose of 200 units/kg/day, insulin aspart increased the incidence of mammary gland tumors in females when compared to untreated controls. The incidence of mammary tumors for insulin aspart was not significantly different than for regular human insulin. The relevance of these findings to humans is not known.
Insulin aspart was not genotoxic in the following tests: Ames test, mouse lymphoma cell forward gene mutation test, human peripheral blood lymphocyte chromosome aberration test, in vivo micronucleus test in mice, and in ex vivo UDS test in rat liver hepatocytes.
In fertility studies in male and female rats, at subcutaneous doses up to 200 units/kg/day (approximately 32 times the human subcutaneous dose, based on units/body surface area), no direct adverse effects on male and female fertility, or general reproductive performance of animals was observed.
-
14 CLINICAL STUDIES 14.1 Overview of Clinical Studies - The efficacy of FIASP was evaluated in 3 randomized, active-controlled trials of 18 to 26 weeks duration in adults and one randomized, active-controlled ...
14.1 Overview of Clinical Studies
The efficacy of FIASP was evaluated in 3 randomized, active-controlled trials of 18 to 26 weeks duration in adults and one randomized, active-controlled, treat-to-target trial of 26 weeks duration in pediatric patients.
In total 1,224 adult subjects (N=763 with type 1 diabetes mellitus; N=461 with type 2 diabetes mellitus) and 519 pediatric subjects with type 1 diabetes mellitus were randomized to FIASP. In adult and pediatric patients with type 1 diabetes mellitus, mealtime FIASP and postmeal FIASP led to non-inferior glycemic control compared to mealtime NovoLog, both in combination with basal insulin. In adult patients with type 2 diabetes mellitus, mealtime FIASP provided non-inferior glycemic control compared to mealtime NovoLog, both in combination with metformin. In addition, mealtime FIASP in a basal-bolus regimen with metformin also provided statistically significant improvement in the overall glycemic control compared to basal insulin therapy alone with metformin in adult patients with type 2 diabetes mellitus.
In adults with type 1 diabetes mellitus (N=472), FIASP led to non-inferior glycemic control compared to NovoLog when both were administered by continuous subcutaneous insulin infusion (CSII) pump.
14.2 Type 1 Diabetes Mellitus - Adults
Study A (NCT01831765): FIASP added to insulin detemir in adult patients with Type 1 Diabetes Mellitus inadequately controlled at baseline.
The efficacy of FIASP was evaluated in a 26-week, randomized, active controlled, treat-to-target, multicenter trial in 1,143 adult patients with type 1 diabetes mellitus inadequately controlled at baseline. Patients were randomized to either blinded mealtime FIASP (N=381), blinded mealtime NovoLog (N=380), or open-label postmeal FIASP (N=382), all in combination with once or twice daily insulin detemir. At randomization, patients were switched to FIASP on a unit to unit basis. Mealtime FIASP or NovoLog was injected 0-2 minutes before the meal, and postmeal FIASP was injected 20 minutes after the start of the meal.
The mean age of the randomized subjects was 44.4 years and mean duration of diabetes was 19.9 years. 59% were male, 93% were White and 2% were Black or African American; and 7% were Hispanic or Latino. The mean BMI was 26.7 kg/m2.
After 26 weeks of treatment, treatment difference in HbA1c reduction from baseline between mealtime FIASP compared to mealtime NovoLog, and the treatment difference between postmeal FIASP compared to mealtime NovoLog met the pre-specified non-inferiority margin (0.4%). See Table 7. Insulin doses were similar among study arms at baseline and at the end of the trial.
Table 7. Results from Study A: 26-Week Trial of Mealtime FIASP and Postmeal FIASP compared to Mealtime NovoLog Used in Combination with Insulin Detemir in Adults with Type 1 Diabetes Mellitus
Mealtime
FIASP+ insulin detemir
Postmeal
FIASP+ insulin detemir
Mealtime
NovoLog+ insulin detemir
Number of subjects randomized (N)
381
382
380
HbA1c (%)
- Baseline (mean)
7.6
7.6
7.6
- Adjusted mean change from baseline
-0.32
-0.13
-0.17
- Estimated treatment difference vs. mealtime NovoLog [95% CI]*
-0.15 [-0.23;-0.07]
0.04 [-0.04;0.12]
Baseline is based on the mean of the observed last available values prior to randomization.
*Tested for non-inferiority
Estimated treatment difference was calculated using mixed model for repeated measurements (MMRM).
7.6% of subjects on the Mealtime FIASP arm, 7.6% of subjects on the Postmeal FIASP arm, and 5.3% of subjects on the Mealtime NovoLog arm were missing the final HbA1c assessment.
14.3 Type 1 Diabetes Mellitus - Pediatric Patients
Study E (NCT02670915): FIASP added to insulin degludec in pediatric patients with Type 1 Diabetes Mellitus.
The efficacy of FIASP was evaluated in a 26-week, randomised, multinational, active controlled, treat-to-target, 3-armed parallel-group trial in 777 pediatric patients with type 1 diabetes mellitus. Patients were randomized to either blinded mealtime FIASP (N=260), blinded mealtime NovoLog (N=258), or open-label postmeal FIASP (N=259), all in combination with once daily insulin degludec. Mealtime FIASP or NovoLog was injected 0-2 minutes before the meal, and postmeal FIASP was injected 20 minutes after the start of the meal.
The mean age of the subjects at baseline was 11.7 years (range 2 to 17 years) and the mean duration of diabetes was 4.4 years. 54% were male, 81% were White, 16% were Asian and 2% were Black or African American. The mean BMI was 19.7 kg/m2.
After 26 weeks of treatment, the treatment difference for change in HbA1c from baseline between mealtime FIASP compared to mealtime NovoLog, and the treatment difference between postmeal FIASP compared to mealtime NovoLog met the pre-specified non-inferiority margin (0.4%). See Table 8. Insulin doses were similar among study arms at baseline and at the end of the trial.
Table 8. Results from Study E: 26-Week Trial of Mealtime FIASP and Postmeal FIASP compared to Mealtime NovoLog Used in Combination with Insulin Degludec in Pediatrics with Type 1 Diabetes Mellitus
Mealtime
FIASP+ insulin degludec
Postmeal
FIASP+ insulin degludec
Mealtime
NovoLog+ insulin degludec
Number of subjects randomized (N)
260
259
258
HbA1c (%)
Baseline (mean)
7.57
7.58
7.53
Adjusted mean change from baseline
0.06
0.35
0.22
Estimated treatment difference vs. mealtime NovoLog [95% CI]*
-0.17 [-0.30; -0.03]
0.13 [-0.01; 0.26]
Baseline is based on the mean of the observed last available values prior to randomization.
*Tested for non-inferiority
Estimated treatment difference was calculated using ANCOVA. Week 26, change in HbA1c was missing for 1.5%, 1.9%, and 1.6% of subjects in mealtime FIASP, post-meal FIASP, and NovoLog respectively. Missing values were imputed with a missing at random assumption.
14.4 Type 2 Diabetes Mellitus - Adults
Study B (NCT01819129): FIASP added to basal insulin and oral antidiabetics in patients with Type 2 Diabetes Mellitus inadequately controlled at baseline on basal insulin and oral antidiabetics
The efficacy of FIASP was evaluated in a 26-week randomized, double-blind, active controlled, treat-to-target, multicenter, multinational, parallel group trial in 689 adult patients with type 2 diabetes mellitus who were inadequately controlled at baseline on basal insulin and oral antidiabetic therapy and had been on these therapies for at least 6 months. Patients were randomized to either mealtime FIASP or to mealtime NovoLog, both in combination with insulin glargine and metformin in a basal-bolus regimen. Mealtime FIASP or mealtime NovoLog was injected 0-2 minutes before the meal.
The mean age of the randomized subjects was 59.5 years and the mean duration of diabetes was 12.7 years. 49% were male, 81% were White, and 6% were Black or African American; and 6% were Hispanic or Latino. The mean BMI was 31.2 kg/m2.
After 26 weeks of treatment, the treatment difference in HbA1c reduction from baseline between mealtime FIASP and mealtime NovoLog, both in combination with insulin glargine and metformin, met the pre-specified non-inferiority margin (0.4%). See Table 9. Insulin doses were similar among study arms at the end of the trial.
Table 9. Results from Study B: 26-Week Trial of Mealtime FIASP Compared to Mealtime NovoLog, Both used in Combination with Insulin Glargine and Metformin, in Adults with Type 2 Diabetes Mellitus
Mealtime
FIASP+insulin glargine
+metformin
Mealtime
NovoLog+insulin glargine
+metformin
Number of subjects randomized (N)
345
344
HbA1c (%)
- Baseline
8.0
7.9
- Adjusted change from baseline
-1.38
-1.36
- Estimated treatment difference
- vs. NovoLog [95%CI]*
-0.02 [-0.15;0.10]
Baseline is based on the mean of the observed last available values prior to randomization.
*Tested for non-inferiority
Estimated treatment difference was calculated using mixed model for repeated measurements (MMRM).
11.9% of subjects on the Mealtime FIASP arm and 10.2% of subjects on the Mealtime NovoLog arm were missing the final HbA1c assessment.
Study C (NCT01850615): FIASP added to basal insulin and metformin in patients with Type 2 Diabetes Mellitus inadequately controlled at baseline on basal insulin and metformin
The efficacy of FIASP was evaluated in an 18-week randomized, open-label, parallel group trial in 236 adult patients with type 2 diabetes mellitus who were inadequately controlled on basal insulin and metformin therapy, either with or without other oral antidiabetic therapy, for at least 3 months. Patients were randomized to either mealtime FIASP in addition to basal insulin and metformin or to continuing basal insulin and metformin therapy without FIASP. The basal insulins used in both treatment arms were insulin glargine, insulin detemir or NPH. All patients were also required to be on ≥1000 mg metformin treatment at baseline.
The mean age of the trial population was 57.4 years and the mean duration of diabetes was 11years. 48% were male, 70% were White and 4% were Black or African American; and 37% were Hispanic or Latino. The mean BMI was 30.8 kg/m2.
After 18 weeks of treatment, addition of FIASP to basal insulin and metformin statistically significantly reduced HbA1c compared to continuing basal insulin and metformin therapy without addition of FIASP (Table 10).
Table 10. Results from Study C: 18-Week Trial of Mealtime FIASP in Adults with Type 2 Diabetes Mellitus Inadequately Controlled at Baseline on Basal Insulin and Metformin
FIASP + basal insulin + metformin
Basal insulin + metformin
Number of subjects randomized (N)
116
120
HbA1c (%)
- Baseline
7.9
7.9
- Adjusted change from baseline
-1.16
-0.22
- Estimated treatment difference
- vs. basal insulin+metformin [95%CI]
-0.94 [-1.17; -0.72]*
Proportion of patients Achieving HbA1c < 7% at Trial End
60.3%
18.3%
Baseline is based on the mean of the observed last available values prior to randomization.
*p<0.0001, 1-sided p-value evaluated at 2.5% level for superiority.
Estimated treatment difference was calculated using mixed model for repeated measurements (MMRM).
6.0% of subjects on the mealtime FIASP arm and 3.3% of subjects on the placebo arm were missing the final HbA1c assessment.
Close14.5 Type 1 Diabetes Mellitus– Adult Continuous Subcutaneous Insulin Infusion (CSII)
Study D (NCT02825251): FIASP in Continuous Subcutaneous Insulin Infusion (CSII) in Adults with Type 1 Diabetes Mellitus
The efficacy and safety of FIASP vs. NovoLog in CSII in adult subjects with type 1 diabetes mellitus (N=472) was evaluated in a randomized, multicenter, multinational, active controlled, treat-to-target, parallel group trial with a 4-week run-in and a 16-week treatment period. Meal-time bolus insulin infusion was initiated 0-2 minutes before a meal.
The mean age of the randomized subjects was 43 years and the mean duration of diabetes was 24 years. 43% were male. 89% were White, 1% were Black or African American, and 1% were Asian; and 3% were Hispanic or Latino. The mean BMI was 26.3 kg/m2.
After 16 weeks of treatment, the treatment difference in HbA1c reduction from baseline between FIASP and NovoLog was 0.10 with 95%CI [0.02, 0.18] (Table 11).
Table 11. Results from Study D: 16-Week Trial FIASP in Adults with Type 1 Diabetes
FIASP
NovoLog
Number of subjects randomized (N)
236
236
HbA1c (%)
- Baseline
7.5
7.5
- Adjusted change from baseline
-0.04
-0.14
- Estimated treatment difference
- FIASP vs. NovoLog [95%CI]*
0.10 [0.02; 0.18]
Proportion of patients Achieving HbA1c < 7% at Trial End
20.3%
23.3%
Baseline is based on the mean of the observed last available values prior to randomization.
*Tested for non-inferiority using a margin of 0.4%.
Estimated treatment difference was calculated using ANCOVA.
2.1% of subjects on the FIASP arm and 2.5% of subjects on the NovoLog arm were missing the final HbA1c assessment. Missing values were imputed using multiple imputation with a mean equal to the baseline value of the corresponding patient.
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - FIASP (insulin aspart) injection 100 units of insulin aspart per mL (U-100) is available as a clear and colorless solution in the following presentations and packaging ...
16.1 How Supplied
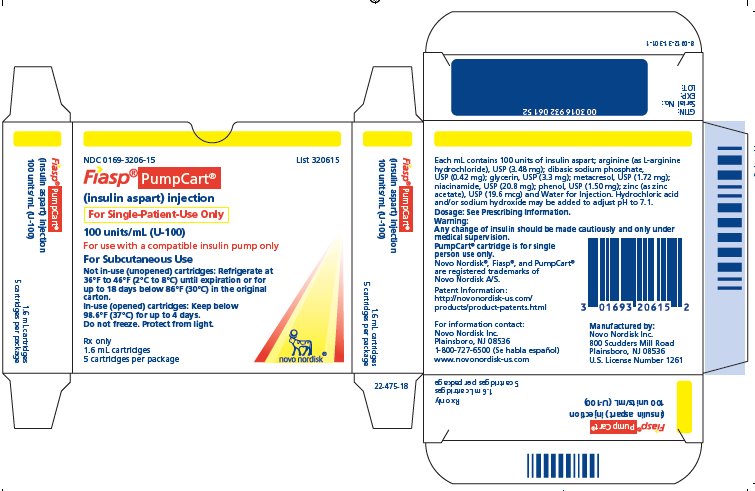
FIASP (insulin aspart) injection 100 units of insulin aspart per mL (U-100) is available as a clear and colorless solution in the following presentations and packaging configurations:
- Carton of one 10 mL multiple-dose vials NDC 0169-3201-11
- Carton of five 3 mL single-patient-use FIASP FlexTouch pens NDC 0169-3204-15
- Carton of five 3 mL single-patient-use PenFill cartridges* NDC 0169-3205-15
- Carton of five 1.6 mL single-patient-use PumpCart cartridges** NDC 0169-3206-15
- The FIASP FlexTouch pen dials in 1 unit increments.
- * FIASP PenFill cartridges are designed for use with Novo Nordisk insulin delivery devices.
- ** FIASP PumpCart cartridges are designed for use in compatible pumps only.
Close16.2 Recommended Storage
Dispense in the original sealed carton with the enclosed Instructions for Use.
Unused FIASP vials should be stored between 2° to 8°C (36° to 46°F) in a refrigerator, but not in or near a freezing compartment. FIASP should not be exposed to excessive heat or light and must never be frozen. Do not use FIASP if it has been frozen. FIASP should not be drawn into a syringe and stored for later use.
Keep the cap on the pen in order to protect from light. Remove the needle from the FIASP FlexTouch pen after each injection and store without a needle attached. Use a new needle for each injection. Keep unused vials, FIASP FlexTouch, PenFill Cartridges and PumpCart cartridges in the carton so they will stay clean and protected from light.
The storage conditions for vials, FIASP FlexTouch pens, 3 mL PenFill cartridges and 1.6 mL PumpCart cartridges are summarized in Table 12:
Table 12. Storage Conditions for Vial, FIASP FlexTouch, PenFill Cartridges
and PumpCart Cartridges
FIASP
presentation
Not in-use (unopened)
In-use (opened)
Room Temperature (up to 30°C [86°F])
Refrigerated
(2°C to 8°C [36°F to 46°F])
Room Temperature (up to 30°C [86°F])
Refrigerated
(2°C to 8°C [36°F to 46°F])
10 mL multiple-dose vial
28 days
Until expiration date
28 days*
(up to 30°C [86°F])
28 days*
3 mL single-patient-use FIASP FlexTouch pen
28 days
Until expiration date
28 days
(up to 30°C [86°F])
28 days
3 mL single-patient-use PenFill cartridges
28 days
Until expiration date
28 days
(up to 30°C [86°F])
Do not refrigerate
1.6 mL single-patient-use PumpCart cartridges
18 days**
Until expiration date
4 days**
(up to 37°C [98.6°F])
Do not refrigerate
*Pump Reservoir: The total in use time is 28 days, including 6 days in the pump reservoir
**PumpCart Cartridges: The maximum time at room temperature is 18 days including 4 days in the pump
Storage of FIASP in Insulin Pump:
- •
- Change FIASP in the pump reservoir at least every 6 days or according to the pump user manual, whichever is shorter.
- •
- Replace the FIASP PumpCart cartridge at least every 4 days or according to the pump user manual, whichever is shorter.
- •
- Change FIASP to avoid insulin degradation or after exposure to temperatures that exceed 37°C (98.6°F).
- •
- The infusion set and infusion set insertion sites should be changed according to the manufacturers’ user manual.
Storage of FIASP in Intravenous Infusion Fluids:
Infusion bags prepared as indicated under Dosage and Administration (2.2) are stable at room temperature at 20°C to 25°C (68°F to 77°F) for 24 hours. -
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-Approved Patient Labeling (Patient Information and Instructions for Use). Never Share a FIASP FlexTouch Pen Device, PenFill Cartridge or PenFill Cartridge ...
Advise the patient to read the FDA-Approved Patient Labeling (Patient Information and Instructions for Use).
Never Share a FIASP FlexTouch Pen Device, PenFill Cartridge or PenFill Cartridge Device Between Patients
Advise patients that they should never share a FIASP FlexTouch pen device, PenFill cartridge or PenFill cartridge devices with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens. Advise patients using FIASP vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.1)].
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of FIASP therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia [see Warnings and Precautions (5.3)].
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery.
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with FIASP. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.6)].
Hypoglycemia due to Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products.
Patients Using Continuous Subcutaneous Insulin Pumps
- •
- Train patients in intensive insulin therapy with multiple injections and in the function of their pump and pump accessories.
- •
- Instruct patients to follow healthcare provider recommendations when setting basal and meal time infusion rate.
- •
- Refer to the continuous subcutaneous infusion pump user manual to see if FIASP can be used with the pump. See recommended reservoir and infusion sets in the insulin pump user manual.
- •
- Instruct patients to change FIASP in the pump reservoir at least every 6 days, or replace the PumpCart cartridge at least every 4 days or according to the pump user manual, whichever is shorter; infusion sets and infusion set insertion sites should be changed in accordance with the manufacturers’ user manual. By following this schedule, patients avoid insulin degradation, infusion set occlusion, and loss of the insulin preservative.
- •
- Instruct patients to discard insulin exposed to temperatures higher than 37°C (98.6°F).
- •
- Instruct patients to inform physician and select a new site for infusion if infusion site becomes erythematous, pruritic, or thickened.
- •
- Instruct patients on the risk of rapid hyperglycemia and ketosis due to pump malfunction, infusion set occlusion, leakage, disconnection or kinking, and degraded insulin. Instruct patients on the risk of hypoglycemia from pump malfunction. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician [see Warnings and Precautions (5) and How Supplied/Storage and Handling (16.2)].
Version: 8
Novo Nordisk®, FIASP®, NovoLog®, FlexTouch®, PenFill® and PumpCart® are registered trademarks of Novo Nordisk A/S.
PATENT Information: http://novonordisk-us.com/products/product-patents.html
© 2023
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
U.S. License 1261
For information about FIASP contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500www.novonordisk-us.com
Close -
Patient Information FIASP® (fee’ asp) (insulin aspart) Injection, for subcutaneous or intravenous use - Do not share your FIASP with other people, even if the needle has been changed. You may give other people a ...
FIASP® (fee’ asp)
(insulin aspart)Injection, for subcutaneous or intravenous use
Do not share your FIASP with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What is FIASP?
- •
- FIASP is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Who should not take FIASP?
Do not take FIASP if you:- •
- are having an episode of low blood sugar (hypoglycemia).
- •
- have an allergy to insulin aspart or any of the ingredients in FIASP.
Before taking FIASP, tell your healthcare provider about all your medical conditions including, if you:
- •
- have kidney problems.
- •
- have liver problems.
- •
- are pregnant or plan to become pregnant. Talk with your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- •
- are breastfeeding or plan to breastfeed. It is not known if FIASP passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while using FIASP.
- •
- are taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking FIASP, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take FIASP?
- •
- Read the Instructions for Use that come with your FIASP.
- •
- Take FIASP exactly as your healthcare provider tells you to.
- •
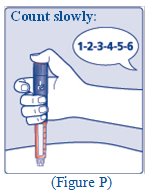
- FIASP starts acting fast. You should take your dose of FIASP at the beginning of the meal or within 20 minutes after starting a meal.
- •
- Know the type and strength of insulin you take. Do not change the type of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- •
- If you miss a dose of FIASP, monitor your blood sugar levels to decide if an insulin dose is needed. Continue with your regular dosing schedule at the next meal.
- •
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- •
- Do not reuse or share needles with other people. You may give other people a serious infection or get a serious infection from them.
- •
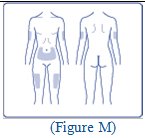
- FIASP can be injected under the skin (subcutaneously) of your stomach area, upper legs, or upper arms, or by continuous infusion under the skin (subcutaneously) through an insulin pump into an area of your body recommended in the instructions that come with your insulin pump.
- •
- Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting pits in skin or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites.
- o
- Do not use the exact same spot for each injection.
- o
- Do not inject where the skin has pits, is thickened, or has lumps.
- o
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking FIASP?
While taking FIASP do not:- •
- Drive or operate heavy machinery until you know how FIASP affects you.
- •
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of FIASP?
FIASP may cause serious side effects that can lead to death, including:
- •
- low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- o
- dizziness or light-headedness
- o
- blurred vision
- o
- anxiety, irritability, or mood changes
- o
- sweating
- o
- slurred speech
- o
- hunger
- o
- confusion
- o
- shakiness
- o
- headache
- o
- fast heart beat
- •
- low potassium in your blood (hypokalemia).
- •
-
serious allergic reactions (whole body reactions). Get emergency medical help right away, if you have any of these signs or symptoms of a severe allergic reaction:
- o
- a rash over your whole body, trouble breathing, a fast heartbeat, swelling of your face, tongue or throat, sweating, extreme drowsiness, dizziness, confusion.
- •
- heart failure. Taking certain diabetes pills called TZDs (thiazolidinediones) with FIASP may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with FIASP. Your healthcare provider should monitor you closely while you are taking TZDs with FIASP. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, sudden weight gain. Treatment with TZDs and FIASP may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Your insulin dose may need to change because of:
- •
- change in level of physical activity or exercise
- •
- increased stress
- •
- change in diet
- •
- weight gain or loss
- •
- illness
Common side effects of FIASP may include:
- •
- skin problems such as eczema, rash, itching, redness and swelling of your skin (dermatitis)
- •
- reactions at the injection site such as itching, rash
- •
- skin thickening or pits at the injection site (lipodystrophy)
- •
- weight gain
These are not all the possible side effects of FIASP. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of FIASP.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about FIASP that is written for health professionals. Do not use FIASP for a condition for which it was not prescribed. Do not give FIASP to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in FIASP?
Active Ingredient: insulin aspart
Inactive Ingredients: arginine, dibasic sodium phosphate, glycerin, metacresol, niacinamide, phenol, zinc and water for injection.
Manufactured by:
Novo Nordisk Inc.U.S. License Number 1261
For more information, go to www.novonordisk-us.com or call 1-800-727-6500.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 06/2023
INSTRUCTIONS FOR USE
FIASP (fee’ asp)
(insulin aspart) injection
10 mL multiple-dose vial (100 units/mL, U-100)
Read this Instructions for Use before you start taking FIASP and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. The vial is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product and insulin syringe.
Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
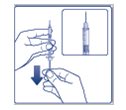
Supplies you will need to give your FIASP injection:
- •
- a 10 mL FIASP vial
- •
- a U-100 insulin syringe and needle
- •
- 2 alcohol swabs
- •
- 1 sharps container for throwing away used needles and syringes. See “Disposing of your used needles and syringes” at the end of these instructions.
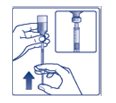
Preparing your FIASP dose:
- •
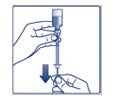
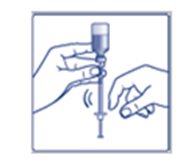
- Do not roll or shake the FIASP vial. Shaking the FIASP vial right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin.
- •
- The tamper-resistant cap should not be loose or damaged before the first use. Do not use if the tamper-resistant cap is loose or damaged before using FIASP for the first time.
- •
- Wash your hands with soap and water.
- •
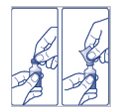
- Before you start to prepare your injection, check the FIASP label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- •
- Check that the FIASP vial is not cracked or damaged. Do not use if the FIASP vial is cracked or damaged.
- •
- FIASP should look clear and colorless. Do not use FIASP if it is thick, cloudy, or is colored.
- •
- Do not use FIASP past the expiration date printed on the label.
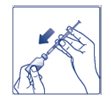
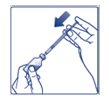
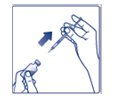
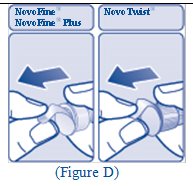
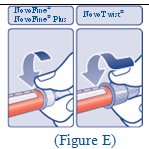
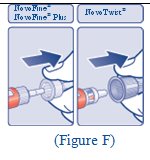
Giving your FIASP injection:
- •
- Inject your FIASP exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.
- •
- FIASP can be injected under the skin (subcutaneously) of your stomach area, upper legs, or upper arms, infused in an insulin pump into an area of your body recommended in the instructions that come with your insulin pump, or given through a needle in your arm (intravenously) by your healthcare provider. Do not inject FIASP into your muscle.
- •
- If you use FIASP in an insulin pump, you should change the infusion sets and the infusion set insertion site according to the pump manufacturers’ user manual. The insulin in the reservoir should be changed at least every 6 days even if you have not used all of the insulin.
- •
- If you use FIASP in an insulin pump, see your insulin pump manual for instructions or talk to your healthcare provider. Your healthcare provider should provide recommendations for appropriate basal and meal time infusion rates.
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- •
- Do not dilute or mix FIASP with any other type of insulin products or solutions.
After your injection:
- •
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
Disposing of your used needles and syringes:
Put your used insulin needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic;
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out;
- o
- upright and stable during use;
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store FIASP?
- •
- Do not freeze FIASP. Do not use FIASP if it has been frozen.
- •
- Keep FIASP away from excessive heat or light.
All unopened vials:
- •
- Store unopened FIASP vials in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
- •
- If unopened vials have been stored in the refrigerator, vials may be used until the expiration date printed on the label.
- •
- If unopened vials have been stored at room temperature, vials should be thrown away after 28 days.
After vials have been opened:
- •
- Opened FIASP vials can be stored in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
- •
- Throw away all opened FIASP vials after 28 days (including 6 days pump in-use time), even if they still have insulin left in them.
General information about the safe and effective use of FIASP
- •
- Always use a new syringe and needle for each injection to help ensure sterility and prevent blocked needles.
- •
- Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
- •
- Keep FIASP vials, syringes, and needles out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
FIASP® is a registered trademark of Novo Nordisk A/S.
PATENT Information: http://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
For information about FIASP® contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
Revised: 06/2023
INSTRUCTIONS FOR USE
FIASP® [fee’ asp]FlexTouch®
(insulin aspart)
injection, for subcutaneous use
3 mL single-patient-use pen: 100 units/mL (U-100)
- •
- Do not share your FIASP FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
- •
- FIASP FlexTouch Pen (“Pen”) is a prefilled disposable, single-patient-use pen containing 300 units of U-100 FIASP (insulin aspart) injection. You can inject from 1 to 80 units in a single injection. The units can be increased by 1 unit at a time.
- •
- People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
- •
- Do not use a syringe to remove FIASP from the FlexTouch Pen.
Supplies you will need to give your FIASP injection:
- •
- FIASP FlexTouch Pen
- •
- a new NovoFine, NovoFine Plus or NovoTwist needle
- •
- alcohol swab
- •
- a sharps container for throwing away used Pens and needles. See “After your injection” at the end of these instructions.
Preparing your FIASP FlexTouch Pen:
- •
- Wash your hands with soap and water.
- •
- Before you start to prepare your injection, check the FIASP FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- •
- FIASP should look clear and colorless. Do not use FIASP if it is thick, cloudy, or is colored.
- •
- Do not use FIASP past the expiration date printed on the label or 28 days after you start using the Pen.
- •
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
Priming your FIASP FlexTouch Pen:
Selecting your dose:
Giving your injection:
- •
- Inject your FIASP exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.
- •
- FIASP can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs) or upper arms. Do not inject FIASP into your muscle.
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
After your injection:
- •
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my FIASP FlexTouch Pen?
Before use:
- •
- Store unused FIASP FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
- •
- Do not freeze FIASP. Do not use FIASP if it has been frozen.
- •
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
- •
- If FIASP FlexTouch Pens are stored at room temperature prior to first use, it should be used or thrown away within 28 days.
Pen in use:
- •
- Store the Pen you are currently using without the needle attached at room temperature below 86°F (30°C) or in the refrigerator at 36°F to 46°F (2°C to 8°C) for up to 28 days.
- •
- Keep FIASP away from excessive heat or light.
- •
- The FIASP FlexTouch Pen you are using is to be thrown away after 28 days, even if it still has insulin left in it and the expiration date has not passed.
General Information about the safe and effective use of FIASP:
- •
- Keep FIASP FlexTouch Pens and needles out of the reach of children.
- •
- Always use a new needle for each injection.
- •
- Do not share FIASP FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
Revised: 06/2023
For more information go to
© 2023 Novo Nordisk
INSTRUCTIONS FOR USE
FIASP® [fee’asp] PenFill®
(insulin aspart)
injection, for subcutaneous use
3 mL cartridge: 100 units/mL (U-100)
- •
- Do not share your PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- •
- Your healthcare provider should show you or your caregiver how to inject FIASP the right way before you inject it for the first time.
- •
- FIASP PenFillcartridge 100 units/mL is a prefilled single-patient-use cartridge containing 300 units of U-100 FIASP (insulin aspart) injection.
- •
- After you insert the PenFill cartridge in your device, you can use it for multiple injections. Read the instruction manual that comes with your insulin delivery device for complete instructions on how to use the PenFill cartridge with the device.
- •
- This PenFill cartridge is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product and your insulin delivery device.
- •
- If using a new FIASP PenFill cartridge, start with Step 1.
- •
- If the FIASP PenFill cartridge has already been used, start with Step 2.
Supplies you will need to give your FIASP injection:
- •
- FIASP PenFill cartridge
- •
- Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device
- •
- 1 new NovoFine®, NovoFine® Plus, or NovoTwist® needle
- •
- Alcohol swab
- •
- Adhesive bandage
- •
- Cotton gauze
- •
- A sharps container for throwing away used PenFill cartridges and needles. See “After your injection” at the end of these instructions.
(Figure A)
How to use the FIASP PenFill cartridge
- •
- Wash your hands with soap and water.
- •
- Before you start to prepare your injection, check the FIASP PenFill cartridge label to make sure that it contains the insulin you need. This is especially important if you take more than 1 type of insulin.
- •
- The tamper-resistant foil should be in place before the first use. If the foil has been broken or removed before your first use of the cartridge, do not use it. Call Novo Nordisk at 1-800-727-6500.
- •
- Carefully look at the cartridge and the insulin inside it. Check that the FIASP cartridge:
- o
- is not damaged, for example cracked or leaking
- o
- is not loose on the threaded end
- •
- FIASP should look clear and colorless. Do not use FIASP if it is cloudy or colored or if the threaded end is loose (See Figure B).
(Figure B)
Step 1:
- •
- Insert a 3 mL cartridge with the threaded end first into your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device (See Figure C).
- •
- If you drop your device, check the insulin cartridge for damage such as cracks or leaking. If your cartridge is damaged, throw it away and use a new one.
(Figure C)
Prepare your device with a new needle
Step 2:
- •
- Take a new needle, and tear off the paper tab. Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not attach a new needle to your device until you are ready to give your injection. Do not reuse or share your needles with other people. You may give others a serious infection, or get a serious infection from them.
- •
- Be careful not to bend or damage the needle before you use it.
- •
- Push the needle straight onto the device. Turn the needle clockwise until it is on tight (See Figure D).
(Figure D)
Step 3:
- •
- Pull off the outer needle cap (See Figure E). Do not throw it away. You will need it after the injection to safely remove the needle.
(Figure E)
Step 4:
- •
- Pull off the inner needle cap and throw it away (See Figure F). Do not try to put the inner needle cap back on the needle.
(Figure F)
A drop of insulin may appear at the needle tip. This is normal, but you must still check the insulin flow.
Check the insulin flow
Step 5:
- •
- Small amounts of air may collect in the cartridge during normal use. You must do an airshot before each injection to avoid injecting air and to make sure you receive the prescribed dose of your medicine.
- •
- Do the airshot as described in the instruction manual that comes with your device.
- •
- Keep testing your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device until you see insulin at the needle tip. If you still do not see a drop of insulin after 6 times, change the needle and repeat this step. This makes sure that any air bubbles are removed and that insulin is getting through the needle (See Figure G).
(Figure G)
Select your dose
Step 6:
- •
- Check to make sure that the dose counter is set to 0.
- •
- Turn the dose selector clockwise to select the dose you need to inject (See Figure H). The pointer should line up with your dose. When turning the dose selector, be careful not to press the dose button as insulin will come out. You will hear a click for every single unit dialed. Do not set the dose by counting the number of clicks you hear because you may get an incorrect dose.
- •
- Refer to your insulin delivery device manual if necessary.
(Figure H)
Inject your dose
Step 7:
- •
- Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.
- •
- FIASP can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs), or upper arms (See Figure I).
(Figure I)
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- •
- Clean your injection site with an alcohol swab. Let your skin dry. Do not touch this area again before injecting.
- •
- Insert the needle into your skin. Press and hold down the dose button until the dose counter shows “0”. Continue to keep the dose button pressed and keep the needle in your skin and slowly count to 6 (see Figure J).
- •
- Remove the needle from your skin.
(Figure J)
You may see a drop of FIASP at the needle tip after injecting. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with a cotton gauze and cover with an adhesive bandage, if necessary. Do not rub the area.
After your injection
Step 8:
- •
- Lay your outer needle cap on a flat surface. Carefully, lead the needle tip into the outer needle cap without touching the needle (See Figure K) and push the outer needle cap completely on.
(Figure K)
- •
- Hold the black cartridge holder on the insulin delivery device and unscrew the needle counterclockwise (See Figure L).
(Figure L)
- •
- Throw away (dispose of) the needle in an FDA-cleared sharps container as your healthcare professional has instructed you.
- •
- Put your empty FIASP PenFill cartridge and used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and PenFill cartridges in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 9:
- •
- Keep the 3 mL PenFill cartridge in the device. Do not store your device with a needle attached. This will prevent infection or leakage of insulin and will make sure that you receive the right dose of FIASP.
- •
- Put the pen cap on your device after each use to protect the insulin from light (See Figure M).
(Figure M)
How should I store my FIASP PenFill cartridge?
Before use:
- •
- Store unused FIASP PenFill cartridges in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Do not freeze FIASP. Do not use FIASP if it has been frozen.
- •
- Unused PenFill cartridges may be used until the expiration date printed on the label, if kept in the refrigerator.
- •
- If FIASP is stored mistakenly outside of refrigeration between 47°F (9°C) to 86°F (30°C) prior to first use, it should be used within 28 days or thrown away.
PenFill cartridges in use:
- •
- Store the PenFill cartridge you are currently using in the insulin delivery device at room temperature below 86°F (30°C) for up to 28 days. Do not refrigerate.
- •
- Keep FIASP away from heat or light.
- •
- The FIASP PenFill cartridge you are using should be thrown away after 28 days, even if it still has insulin left in it.
General Information about the safe and effective use of FIASP.
- •
- Keep FIASP PenFill cartridges and needles out of the reach of children.
- •
- Do not share FIASP PenFill cartridges or needles with other people. You may give other people a serious infection, or get a serious infection from them.
- •
- Always carry extra insulin of the same type(s) you use in case of loss or damage.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
Revised: 06/2023
CloseINSTRUCTIONS FOR USE
FIASP®[fee’asp] PumpCart®
(insulin aspart)
injection, for subcutaneous use
1.6 mL cartridge: 100 units/mL (U-100)
- •
- Please read the pump manual (user guide) that comes with your insulin pump.
- •
- Only use FIASP PumpCart with a compatible insulin pump. Check the insulin pump manual for instructions to see if FIASP PumpCart can be used with the pump. Do not use with other devices that are not designed for use with FIASP PumpCart.
- •
- FIASP PumpCart is ready for use directly in the pump. Using the wrong device may result in the wrong insulin dosing and lead to high blood sugar (hyperglycemia) or low blood sugar (hypoglycemia).
- •
- Do not share your PumpCart cartridge with other people. You may give other people a serious infection or get a serious infection from them.
- •
- Do not use the PumpCart cartridge if you are blind or visually impaired without the assistance of a person trained in the proper use of the PumpCart.
- •
- Do not mix with any other insulins.
- •
- Do not refill FIASP PumpCart. When it is empty, throw away (dispose) the cartridge.
- •
- Do not use FIASP PumpCart in an insulin pen.
Preparing and Using the FIASP PumpCart cartridge
- •
- Take FIASP PumpCart out of its package.
- •
- Check the label to make sure that it is FIASP PumpCart and confirm that you are using a compatible insulin pump.
- •
- Check the expiry date (EXP) – which is on the label and the carton.
- •
- See picture A. Do not use the cartridge if any damage or leakage is seen, if the plunger has moved, or if the bottom of the plunger is visible above the white label band. This could be a result of leakage of insulin. Contact Novo Nordisk If you suspect that your FIASP PumpCart cartridge is damaged.
- •
- Check that the insulin in FIASP PumpCart is clear and colorless. If the insulin looks cloudy, do not use FIASP PumpCart. The cartridge may contain small bubbles.
- •
- Wash your hands with soap and water prior to installing the cartridge in your insulin pump.
- •
- Follow the instructions in the pump user manual to insert a new FIASP PumpCart cartridge in your pump and to remove the FIASP PumpCart cartridge from your pump.
- •
- Replace the FIASP PumpCart cartridge at least every 4 days, or according to the pump user manual, whichever is shorter, even if you have not used all of the insulin.
- •
- Change the infusion sets and the infusion set insertion site according to the insulin pump manufacturers’ user manual.
- •
- Throw away FIASP PumpCart in the pump reservoir if it has been exposed to temperatures higher than 98.6°F (37°C).
- How should I dispose my FIASP PumpCart cartridge?
- •
- Put your empty FIASP PumpCart in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) Fiasp PumpCart in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal. Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my FIASP PumpCart cartridge?
- •
- Not in-use (unopened) cartridges: Refrigerate at 36°F to 46°F (2°C to 8°C) in the original carton until expiration or for up to 18 days below 86°F (30°C).
- •
- In-use (opened) cartridge: Do not refrigerate. Keep below 98.6°F (37°C) for up to 4 days. The maximum time at room temperature is 18 days including 4 days in the pump.
- •
- Do not freeze.
- •
- Keep FIASP PumpCart cartridge away from heat or light.
General Information about the safe and effective use of FIASP PumpCart cartridge
- •
- Keep FIASP PumpCart out of the reach of children.
- •
- Always carry extra insulin of the same type you use in case of loss or damage.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
US License Number 1261
Revised: 06/2023
-
PRINCIPAL DISPLAY PANEL - VIAL NDC 0169-3201-11 List 320111 - FIASP® (insulin apart) injection - 100 units/mL (U-100) For subcutaneous or intravenous use - Rx only One 10 mL multi-dose vial
NDC 0169-3201-11 List 320111
FIASP®
(insulin apart) injection
100 units/mL (U-100)
For subcutaneous or intravenous use
- Rx only One 10 mL multi-dose vial
-
PRINCIPAL DISPLAY PANEL - FLEXTOUCH NDC 0169-3204-15 List 320415 - FIASP®FlexTouch® (insulin aspart) injection - For Single-Patient Use Only - 100 units/mL (U-100) For subcutaneous use only - Rx Only - Recommended for use ...
NDC 0169-3204-15 List 320415
FIASP®FlexTouch®
(insulin aspart) injection
For Single-Patient Use Only
100 units/mL (U-100)
For subcutaneous use only
Rx Only
Recommended for use with NovoFine® or
NovoFine® Plus disposable needles.
Keep in a refrigerator at 36° to 46°F (2° to 8°C) until first use.
After first use store at 36° to 86°F (2° to 30°C) up to 28 days.
Do not freeze.
Protect from light.
Dispense in this sealed carton.
5×3 mL Prefilled Pens
Close -
PRINCIPAL DISPLAY PANEL - PenFill NDC 0169-3205-15 List 320515 - FIASP® PenFill® (insulin aspart) injection - For Single-Patient Use Only - 100 units/mL (U-100) For Subcutaneous Use - For use with Novo Nordisk 3 mL PenFill ...
- NDC 0169-3205-15 List 320515
FIASP® PenFill®
(insulin aspart) injection
For Single-Patient Use Only
100 units/mL (U-100)
For Subcutaneous Use
For use with Novo Nordisk 3 mL PenFill® cartridge compatible delivery devices, NovoPen® Echo®, and NovoFine®/NovoFine® Plus disposable needles.
Not in-use (unopened) cartridges: Refrigerate at 36°F to 46°F (2°C to 8°C) in the original carton. In-use (opened) cartridge: Keep at room temperature below 86°F (30°C). Once stored at room temperature, discard after 28 days. Do not freeze. Protect from light.
Rx only
3 mL cartridges
5 cartridges per package
Close -
Principal Display Panel - PumpCart NDC 0169-3206-15 List 320615 - Fiasp® PumpCart® (insulin aspart) injection For Single-Patient Use Only - 100 units/mL (U-100) For use with a compatible ...
NDC 0169-3206-15 List 320615
- Fiasp® PumpCart®
(insulin aspart) injection
For Single-Patient Use Only
100 units/mL (U-100)
For use with a compatible insulin pump only
For Subcutaneous Use
Not in-use (unopened) cartridges: Refrigerate at 36°F to 46°F (2°C to 8°C) until expiration or for up to 18 days below 86°F (30°C) in the original carton.
In-use (opened) cartridges: Keep below 98.6°F (37°C) for up to 4 days.
Do not freeze. Protect from light.
Rx only
1.6 mL cartridges
5 cartridges per package
Close -
INGREDIENTS AND APPEARANCEProduct Information
FIASP insulin aspart injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0169-3201 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN ASPART (UNII: D933668QVX) (INSULIN ASPART - UNII:D933668QVX) INSULIN ASPART 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain HYDROCHLORIC ACID (UNII: QTT17582CB) May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-3201-11 1 in 1 CARTON 10/20/2017 1 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC:0169-3201-90 1 in 1 CARTON 10/20/2017 2 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA208751 10/20/2017 FIASP insulin aspart injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0169-3204 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN ASPART (UNII: D933668QVX) (INSULIN ASPART - UNII:D933668QVX) INSULIN ASPART 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.42 mg in 1 mL WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain HYDROCHLORIC ACID (UNII: QTT17582CB) May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-3204-15 5 in 1 CARTON 10/20/2017 1 3 mL in 1 SYRINGE, PLASTIC; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0169-3204-97 1 in 1 CARTON 10/20/2017 2 NDC:0169-3204-90 3 mL in 1 SYRINGE, PLASTIC; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA208751 10/20/2017 FIASP insulin aspart injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0169-3205 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN ASPART (UNII: D933668QVX) (INSULIN ASPART - UNII:D933668QVX) INSULIN ASPART 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.42 mg in 1 mL WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain HYDROCHLORIC ACID (UNII: QTT17582CB) May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-3205-15 5 in 1 CARTON 10/01/2019 1 NDC:0169-3205-11 3 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0169-3205-95 5 in 1 CARTON 10/01/2019 2 NDC:0169-3205-91 3 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA208751 10/20/2017 FIASP insulin aspart injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0169-3206 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN ASPART (UNII: D933668QVX) (INSULIN ASPART - UNII:D933668QVX) INSULIN ASPART 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain HYDROCHLORIC ACID (UNII: QTT17582CB) May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-3206-15 5 in 1 CARTON 09/14/2023 1 NDC:0169-3206-11 1.6 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0169-3206-95 5 in 1 CARTON 09/14/2023 2 NDC:0169-3206-91 1.6 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA208751 10/20/2017
CloseLabeler - Novo Nordisk (622920320)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
FIASP- insulin aspart injection injection, solution
FIASP- insulin aspart injection, solution
Number of versions: 15
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jul 25, 2023 | 18 (current) | download |
| Sep 9, 2022 | 16 | download |
| Apr 21, 2022 | 15 | download |
| Aug 17, 2020 | 14 | download |
| Jun 15, 2020 | 13 | download |
| Jan 3, 2020 | 12 | download |
| Nov 22, 2019 | 11 | download |
| Nov 21, 2019 | 10 | download |
| Oct 31, 2019 | 9 | download |
| May 23, 2019 | 8 | download |
| Apr 2, 2019 | 7 | download |
| Oct 23, 2018 | 6 | download |
| Sep 11, 2018 | 4 | download |
| Jul 31, 2018 | 3 | download |
| Oct 23, 2017 | 2 | download |
RxNorm
FIASP- insulin aspart injection injection, solution
FIASP- insulin aspart injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 311040 | insulin aspart 100 UNT/ML Injectable Solution | PSN |
| 2 | 311040 | insulin aspart, human 100 UNT/ML Injectable Solution | SCD |
| 3 | 311040 | insulin analog, aspart 100 UNT/ML Injectable Solution | SY |
| 4 | 1653196 | insulin aspart 100 UNT/ML in 3 ML Cartridge | PSN |
| 5 | 1653196 | 3 ML insulin aspart, human 100 UNT/ML Cartridge | SCD |
| 6 | 1653196 | insulin, aspart, human 100 UNT/ML per 3 ML Cartridge | SY |
| 7 | 1653202 | insulin aspart 100 UNT/ML in 3 ML Pen Injector | PSN |
| 8 | 1653202 | 3 ML insulin aspart, human 100 UNT/ML Pen Injector | SCD |
| 9 | 1653202 | insulin, aspart, human 100 UNT/ML per 3 ML Pen Injector | SY |
| 10 | 1986354 | Fiasp 100 UNT/mL Injectable Solution | PSN |
| 11 | 1986354 | insulin aspart, human 100 UNT/ML Injectable Solution [Fiasp] | SBD |
| 12 | 1986354 | Fiasp 100 UNT/ML Injectable Solution | SY |
| 13 | 1986356 | Fiasp 100 UNT/mL in 3 mL Pen Injector | PSN |
| 14 | 1986356 | 3 ML insulin aspart, human 100 UNT/ML Pen Injector [Fiasp] | SBD |
| 15 | 1986356 | 3 ML Fiasp 100 UNT/ML Pen Injector | SY |
| 16 | 2205454 | Fiasp 100 UNT/mL in 3 mL Cartridge | PSN |
| 17 | 2205454 | 3 ML insulin aspart, human 100 UNT/ML Cartridge [Fiasp] | SBD |
| 18 | 2205454 | 3 ML Fiasp 100 UNT/ML Cartridge | SY |
| 19 | 2205454 | Fiasp 100 UNT/ML per 3 ML Cartridge | SY |
| 20 | 2642808 | insulin aspart, human 100 UNT/ML in 1.6 ML Cartridge | PSN |
| 21 | 2642808 | 1.6 ML insulin aspart, human 100 UNT/ML Cartridge | SCD |
| 22 | 2642808 | insulin aspart, human 100 UNT/ML per 1.6 ML Cartridge | SY |
| 23 | 2642809 | Fiasp 100 UNT/mL in 1.6 mL Cartridge | PSN |
| 24 | 2642809 | 1.6 ML insulin aspart, human 100 UNT/ML Cartridge [Fiasp] | SBD |
| 25 | 2642809 | Fiasp 100 UNT/ML per 1.6 ML Cartridge | SY |
Get Label RSS Feed for this Drug
FIASP- insulin aspart injection injection, solution
FIASP- insulin aspart injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=834e7efc-393f-4c55-9125-628562a8a5cf
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
FIASP- insulin aspart injection injection, solution
FIASP- insulin aspart injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0169-3201-11 |
| 2 | 0169-3201-90 |
| 3 | 0169-3204-15 |
| 4 | 0169-3204-90 |
| 5 | 0169-3204-97 |
| 6 | 0169-3205-11 |
| 7 | 0169-3205-15 |
| 8 | 0169-3205-91 |
| 9 | 0169-3205-95 |
| 10 | 0169-3206-11 |
| 11 | 0169-3206-15 |
| 12 | 0169-3206-91 |
| 13 | 0169-3206-95 |