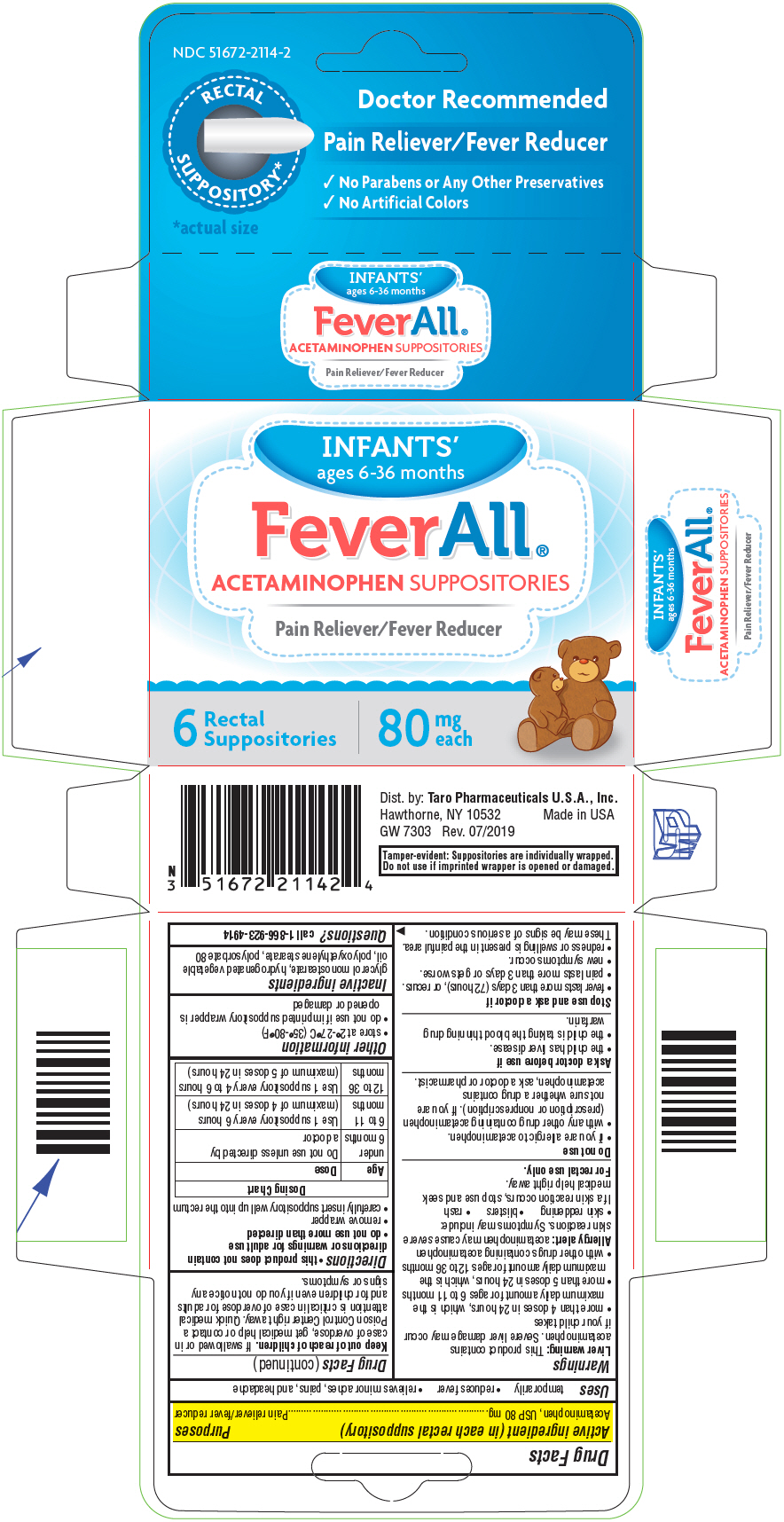
Label: FEVERALL INFANTS- acetaminophen suppository
- NDC Code(s): 51672-2114-0, 51672-2114-2, 51672-2114-4
- Packager: Taro Pharmaceuticals U.S.A. Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each rectal suppository)Acetaminophen, USP 80 mg
-
PurposesPain reliever/fever reducer
-
Usestemporarily - reduces fever - relieves minor aches, pains, and headache
-
WarningsLiver warning - This product contains acetaminophen. Severe liver damage may occur if your child takes - more than 4 doses in 24 hours, which is the maximum daily amount for ages 6 to 11 ...
-
Directionsthis product does not contain directions or warnings for adult use - do not use more than directed - remove wrapper - carefully insert suppository well up into the rectum - Dosing ...
-
Other informationstore at 2°-27°C (35°-80°F) do not use if imprinted suppository wrapper is opened or damaged
-
Inactive ingredientsglycerol monostearate, hydrogenated vegetable oil, polyoxyethylene stearate, polysorbate 80
-
Questions?call 1-866-923-4914
-
SPL UNCLASSIFIED SECTIONDist. by: Taro Pharmaceuticals U.S.A., Inc. Hawthorne, NY 10532
-
PRINCIPAL DISPLAY PANEL - 6 Suppository Blister Pack CartonNDC 51672-2114-2 - RECTAL - SUPPOSITORY* *actual size - Doctor Recommended - Pain Reliever/Fever Reducer - No Parabens or Any Other Preservatives - No Artificial Colors - INFANTS' ages 6-36 ...
-
INGREDIENTS AND APPEARANCEProduct Information