Label: FERAHEME- ferumoxytol injection
- NDC Code(s): 59338-775-01, 59338-775-10
- Packager: AMAG Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Feraheme safely and effectively. See full prescribing information for Feraheme. Feraheme® (ferumoxytol injection), for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS
Fatal and serious hypersensitivity reactions including anaphylaxis have occurred in patients receiving Feraheme. Initial symptoms may include hypotension, syncope, unresponsiveness, cardiac/cardiorespiratory arrest.
- •
- Only administer Feraheme as an intravenous infusion over at least 15 minutes and only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions. [see Warnings and Precautions (5.1)].
- •
- Observe for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following Feraheme infusion including monitoring of blood pressure and pulse during and after Feraheme administration [see Warnings and Precautions (5.1)].
- •
- Hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGEFeraheme is indicated for the treatment of iron deficiency anemia (IDA) in adult patients: • who have intolerance to oral iron or have had unsatisfactory response to oral iron or - • who have ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of Feraheme is an initial 510 mg dose followed by a second 510 mg dose 3 to 8 days later. Administer Feraheme as an intravenous infusion in 50-200 mL 0.9% Sodium Chloride ...
-
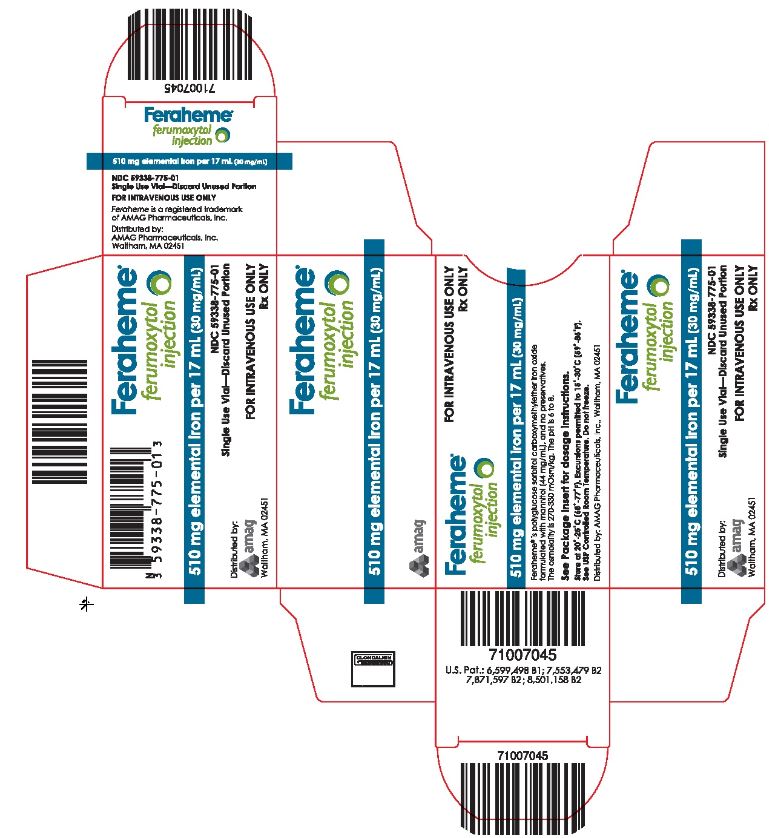
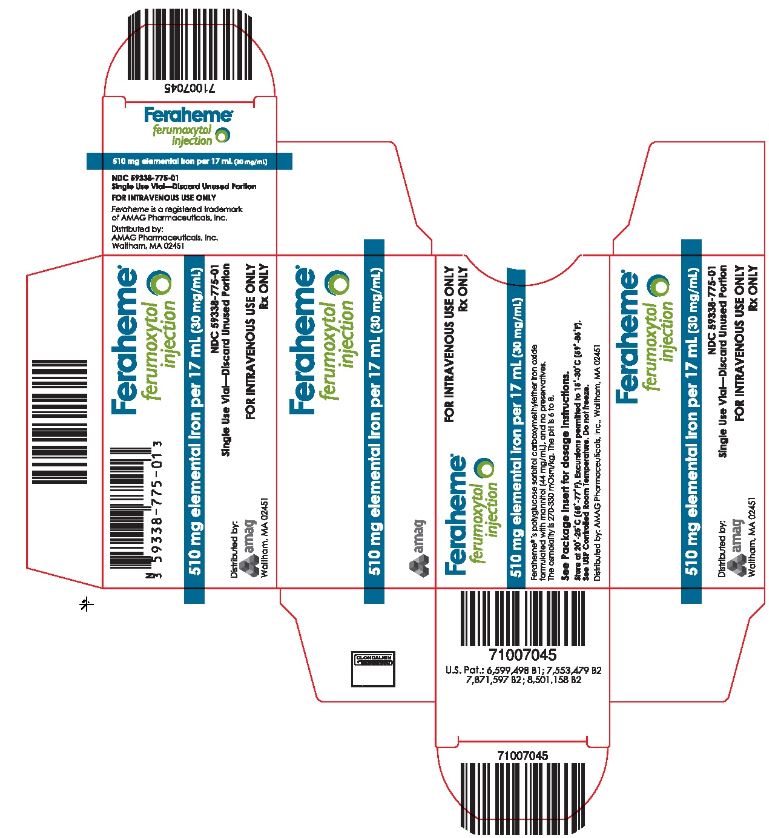
3 DOSAGE FORMS AND STRENGTHSFeraheme Injection is available in single-dose vials. Each vial contains 510 mg of elemental iron in 17 mL (30 mg per mL).
-
4 CONTRAINDICATIONSFeraheme is contraindicated in patients with: • Known hypersensitivity to Feraheme or any of its components [see Warnings and Precautions (5.1)] • History of allergic reaction to any intravenous ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Hypersensitivity Reactions - Fatal and serious hypersensitivity reactions including anaphylaxis, presenting with cardiac/ cardiorespiratory arrest, clinically significant ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Serious Hypersensitivity Reactions [see Warnings and Precautions (5.1)] • Hypotension [see ...
-
7 DRUG INTERACTIONSDrug-drug interaction studies with Feraheme were not conducted. Feraheme may reduce the absorption of concomitantly administered oral iron preparations.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with ferumoxytol use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. There are risks ...
-
10 OVERDOSAGELimited data are available regarding overdosage of Feraheme in humans. Excessive dosages of Feraheme may lead to accumulation of iron in storage sites potentially leading to hemosiderosis. Do not ...
-
11 DESCRIPTIONFeraheme is an iron replacement product containing ferumoxytol for intravenous infusion. Ferumoxytol is a non-stoichiometric magnetite (superparamagnetic iron oxide) coated with polyglucose ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Feraheme consists of a superparamagnetic iron oxide that is coated with a carbohydrate shell, which helps to isolate the bioactive iron from plasma components until ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ferumoxytol was not tested for carcinogenic effects. In standard genotoxicity tests, ferumoxytol showed no evidence of mutagenic ...
-
14 CLINICAL STUDIES14.1 Iron Deficiency Anemia in Patients Who Are Intolerant to Oral Iron or Have Had Unsatisfactory Response to Oral Iron - IDA-301 Trial (referred to as IDA Trial 1) (NCT 01114139), IDA-302 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Feraheme is available in single-dose vials in the following package sizes (Table 6). Table 6: Feraheme Packaging Description - NDC Code - Dose / Total volume per ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Prior History of Allergies to Parenteral Iron Products - Question patients regarding any prior history of ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 02/2018 - Patient Information - Feraheme (FER-uh-heem) (ferumoxytol ...
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – 17 mL Vial, Feraheme Injection
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Carton for Single Use Vial, Feraheme Injection
-
INGREDIENTS AND APPEARANCEProduct Information