Label: FELBATOL- felbamate tablet
FELBATOL- felbamate suspension
- NDC Code(s): 0037-0430-01, 0037-0431-01, 0037-0442-17, 0037-0442-67
- Packager: Meda Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 14, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
1. APLASTIC ANEMIA
THE USE OF FELBATOL® (felbamate) IS ASSOCIATED WITH A MARKED INCREASE IN THE INCIDENCE OF APLASTIC ANEMIA. ACCORDINGLY, FELBATOL® SHOULD ONLY BE USED IN PATIENTS WHOSE EPILEPSY IS SO SEVERE THAT THE RISK OF APLASTIC ANEMIA IS DEEMED ACCEPTABLE IN LIGHT OF THE BENEFITS CONFERRED BY ITS USE (SEE INDICATIONS). ORDINARILY, A PATIENT SHOULD NOT BE PLACED ON AND/OR CONTINUED ON FELBATOL® WITHOUT CONSIDERATION OF APPROPRIATE EXPERT HEMATOLOGIC CONSULTATION.AMONG FELBATOL® TREATED PATIENTS, APLASTIC ANEMIA (PANCYTOPENIA IN THE PRESENCE OF A BONE MARROW LARGELY DEPLETED OF HEMATOPOIETIC PRECURSORS) OCCURS AT AN INCIDENCE THAT MAY BE MORE THAN A 100 FOLD GREATER THAN THAT SEEN IN THE UNTREATED POPULATION (I.E., 2 TO 5 PER MILLION PERSONS PER YEAR). THE RISK OF DEATH IN PATIENTS WITH APLASTIC ANEMIA GENERALLY VARIES AS A FUNCTION OF ITS SEVERITY AND ETIOLOGY; CURRENT ESTIMATES OF THE OVERALL CASE FATALITY RATE ARE IN THE RANGE OF 20 TO 30%, BUT RATES AS HIGH AS 70% HAVE BEEN REPORTED IN THE PAST.
THERE ARE TOO FEW FELBATOL® ASSOCIATED CASES, AND TOO LITTLE KNOWN ABOUT THEM TO PROVIDE A RELIABLE ESTIMATE OF THE SYNDROME'S INCIDENCE OR ITS CASE FATALITY RATE OR TO IDENTIFY THE FACTORS, IF ANY, THAT MIGHT CONCEIVABLY BE USED TO PREDICT WHO IS AT GREATER OR LESSER RISK.
IN MANAGING PATIENTS ON FELBATOL®, IT SHOULD BE BORNE IN MIND THAT THE CLINICAL MANIFESTATION OF APLASTIC ANEMIA MAY NOT BE SEEN UNTIL AFTER A PATIENT HAS BEEN ON FELBATOL® FOR SEVERAL MONTHS (E.G., ONSET OF APLASTIC ANEMIA AMONG FELBATOL® EXPOSED PATIENTS FOR WHOM DATA ARE AVAILABLE HAS RANGED FROM 5 TO 30 WEEKS). HOWEVER, THE INJURY TO BONE MARROW STEM CELLS THAT IS HELD TO BE ULTIMATELY RESPONSIBLE FOR THE ANEMIA MAY OCCUR WEEKS TO MONTHS EARLIER. ACCORDINGLY, PATIENTS WHO ARE DISCONTINUED FROM FELBATOL® REMAIN AT RISK FOR DEVELOPING ANEMIA FOR A VARIABLE, AND UNKNOWN, PERIOD AFTERWARDS.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING APLASTIC ANEMIA CHANGES WITH DURATION OF EXPOSURE. CONSEQUENTLY, IT IS NOT SAFE TO ASSUME THAT A PATIENT WHO HAS BEEN ON FELBATOL® WITHOUT SIGNS OF HEMATOLOGIC ABNORMALITY FOR LONG PERIODS OF TIME IS WITHOUT RISK.
IT IS NOT KNOWN WHETHER OR NOT THE DOSE OF FELBATOL® AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
IT IS NOT KNOWN WHETHER OR NOT CONCOMITANT USE OF ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
APLASTIC ANEMIA TYPICALLY DEVELOPS WITHOUT PREMONITORY CLINICAL OR LABORATORY SIGNS, THE FULL BLOWN SYNDROME PRESENTING WITH SIGNS OF INFECTION, BLEEDING, OR ANEMIA. ACCORDINGLY, ROUTINE BLOOD TESTING CANNOT BE RELIABLY USED TO REDUCE THE INCIDENCE OF APLASTIC ANEMIA, BUT, IT WILL, IN SOME CASES, ALLOW THE DETECTION OF THE HEMATOLOGIC CHANGES BEFORE THE SYNDROME DECLARES ITSELF CLINICALLY. FELBATOL® SHOULD BE DISCONTINUED IF ANY EVIDENCE OF BONE MARROW DEPRESSION OCCURS.
2. HEPATIC FAILURE
EVALUATION OF POSTMARKETING EXPERIENCE SUGGESTS THAT ACUTE LIVER FAILURE IS ASSOCIATED WITH THE USE OF FELBATOL®. THE REPORTED RATE IN THE U.S. HAS BEEN ABOUT 6 CASES OF LIVER FAILURE LEADING TO DEATH OR TRANSPLANT PER 75,000 PATIENT YEARS OF USE. THIS RATE IS AN UNDERESTIMATE BECAUSE OF UNDER REPORTING, AND THE TRUE RATE COULD BE CONSIDERABLY GREATER THAN THIS. FOR EXAMPLE, IF THE REPORTING RATE IS 10%, THE TRUE RATE WOULD BE ONE CASE PER 1,250 PATIENT YEARS OF USE.OF THE CASES REPORTED, ABOUT 67% RESULTED IN DEATH OR LIVER TRANSPLANTATION, USUALLY WITHIN 5 WEEKS OF THE ONSET OF SIGNS AND SYMPTOMS OF LIVER FAILURE. THE EARLIEST ONSET OF SEVERE HEPATIC DYSFUNCTION FOLLOWED SUBSEQUENTLY BY LIVER FAILURE WAS 3 WEEKS AFTER INITIATION OF FELBATOL®. ALTHOUGH SOME REPORTS DESCRIBED DARK URINE AND NONSPECIFIC PRODROMAL SYMPTOMS (E.G., ANOREXIA, MALAISE, AND GASTROINTESTINAL SYMPTOMS), IN OTHER REPORTS IT WAS NOT CLEAR IF ANY PRODROMAL SYMPTOMS PRECEDED THE ONSET OF JAUNDICE.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING HEPATIC FAILURE CHANGES WITH DURATION OF EXPOSURE.
IT IS NOT KNOWN WHETHER OR NOT THE DOSAGE OF FELBATOL® AFFECTS THE INCIDENCE OF HEPATIC FAILURE.
IT IS NOT KNOWN WHETHER CONCOMITANT USE OF OTHER ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECT THE INCIDENCE OF HEPATIC FAILURE.
FELBATOL® SHOULD NOT BE PRESCRIBED FOR ANYONE WITH A HISTORY OF HEPATIC DYSFUNCTION.
TREATMENT WITH FELBATOL® SHOULD BE INITIATED ONLY IN INDIVIDUALS WITHOUT ACTIVE LIVER DISEASE AND WITH NORMAL BASELINE SERUM TRANSAMINASES. IT HAS NOT BEEN PROVED THAT PERIODIC SERUM TRANSAMINASE TESTING WILL PREVENT SERIOUS INJURY BUT IT IS GENERALLY BELIEVED THAT EARLY DETECTION OF DRUG-INDUCED HEPATIC INJURY ALONG WITH IMMEDIATE WITHDRAWAL OF THE SUSPECT DRUG ENHANCES THE LIKELIHOOD FOR RECOVERY. THERE IS NO INFORMATION AVAILABLE THAT DOCUMENTS HOW RAPIDLY PATIENTS CAN PROGRESS FROM NORMAL LIVER FUNCTION TO LIVER FAILURE, BUT OTHER DRUGS KNOWN TO BE HEPATOTOXINS CAN CAUSE LIVER FAILURE RAPIDLY (E.G., FROM NORMAL ENZYMES TO LIVER FAILURE IN 2-4 WEEKS). ACCORDINGLY, MONITORING OF SERUM TRANSAMINASE LEVELS (AST AND ALT) IS RECOMMENDED AT BASELINE AND PERIODICALLY THEREAFTER. WHILE THE MORE FREQUENT THE MONITORING THE GREATER THE CHANCES OF EARLY DETECTION, THE PRECISE SCHEDULE FOR MONITORING IS A MATTER OF CLINICAL JUDGEMENT.
FELBATOL® SHOULD BE DISCONTINUED IF EITHER SERUM AST OR SERUM ALT LEVELS BECOME INCREASED ≥ 2 TIMES THE UPPER LIMIT OF NORMAL, OR IF CLINICAL SIGNS AND SYMPTOMS SUGGEST LIVER FAILURE (SEE PRECAUTIONS). PATIENTS WHO DEVELOP EVIDENCE OF HEPATOCELLULAR INJURY WHILE ON FELBATOL® AND ARE WITHDRAWN FROM THE DRUG FOR ANY REASON SHOULD BE PRESUMED TO BE AT INCREASED RISK FOR LIVER INJURY IF FELBATOL® IS REINTRODUCED. ACCORDINGLY, SUCH PATIENTS SHOULD NOT BE CONSIDERED FOR RE-TREATMENT.
Close -
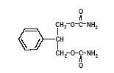
DESCRIPTIONFelbatol® (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol ...
-
CLINICAL PHARMACOLOGYMechanism of Action: The mechanism by which felbamate exerts its anticonvulsant activity is unknown, but in animal test systems designed to detect anticonvulsant activity, felbamate has ...
-
CLINICAL STUDIESThe results of controlled clinical trials established the efficacy of Felbatol® (felbamate) as monotherapy and adjunctive therapy in adults with partial-onset seizures with or without secondary ...
-
INDICATIONS AND USAGEFelbatol® is not indicated as a first line antiepileptic treatment (see Warnings). Felbatol® is recommended for use only in those patients who respond inadequately to alternative treatments and ...
-
CONTRAINDICATIONSFelbatol® is contraindicated in patients with known hypersensitivity to Felbatol®, its ingredients, or known sensitivity to other carbamates. It should not be used in patients with a history of ...
-
WARNINGSSee Boxed Warning regarding aplastic anemia and hepatic failure. Antiepileptic drugs should not be suddenly discontinued because of the possibility of increasing seizure frequency. Suicidal ...
-
PRECAUTIONSDosage Adjustment in the Renally Impaired: A study in otherwise healthy individuals with renal dysfunction indicated that prolonged half-life and reduced clearance of felbamate are ...
-
ADVERSE REACTIONS To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-800-526-3840 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . The most common adverse reactions seen in ...
-
DRUG ABUSE AND DEPENDENCEAbuse: Abuse potential was not evaluated in human studies. Dependence: Rats administered felbamate orally at doses 8.3 times the recommended human dose 6 days each week for 5 ...
-
OVERDOSAGEFour subjects inadvertently received Felbatol® (felbamate) as adjunctive therapy in dosages ranging from 5400 to 7200 mg/day for durations between 6 and 51 days. One subject who received 5400 ...
-
DOSAGE AND ADMINISTRATIONFelbatol® (felbamate) has been studied as monotherapy and adjunctive therapy in adults and as adjunctive therapy in children with seizures associated with Lennox-Gastaut syndrome. As Felbatol ...
-
HOW SUPPLIEDFelbatol® (felbamate) Tablets, 400 mg, are yellow, scored, capsule-shaped tablets, debossed 0430 on one side and FELBATOL 400 on the other; available in bottles of 100 (NDC 0037-0430-01) ...
-
PATIENT/PHYSICIAN ACKNOWLEDGMENT FORM FELBATOL® (felbamate) SHOULD NOT BE USED BY PATIENTS UNTIL THERE HAS BEEN A COMPLETE DISCUSSION OF THE RISKS. All patients treated with Felbatol should acknowledge that they understand the risks ...
-
Medication GuideFELBATOL (Fel-ba-taal) (felbamate) Tablets and Oral Suspension - IS-00431-02 Rev. 2/2018 - SOV-IS-00431-02 Rev. 2/2018 - Read this Medication Guide before you start taking FELBATOL and ...
-
Package Label - Principal Display Panel – 100-count Bottle, 400 mg Tablet NDC 0037-0430-01 - 100 Tablets - Felbatol® (felbamate) Tablets 400 mg - Rx Only - MEDA - Meda Pharmaceuticals® Somerset, New Jersey 08873-4120 - Usual Dosage: For full prescribing ...
-
Package Label - Principal Display Panel – 100-count Bottle, 600 mg Tablet NDC 0037-0431-01 - 100 Tablets - Felbatol® (felbamate) Tablets 600 mg - Rx Only - MEDA - Meda Pharmaceuticals® Somerset, New Jersey 08873-4120 - Usual Dosage: For full prescribing ...
-
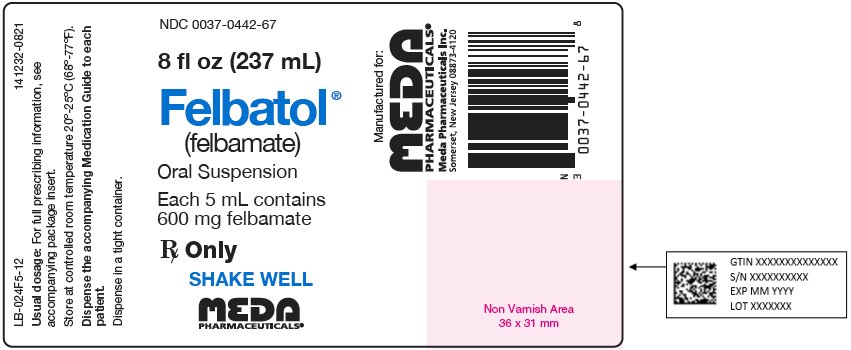
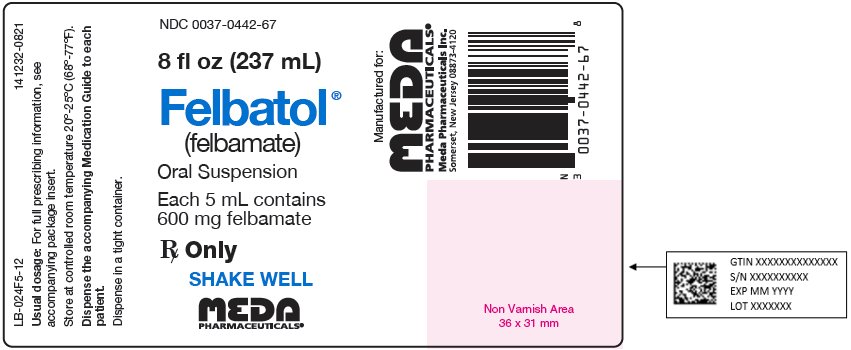
Package Label - Principal Display Panel – 8 fl oz (237 mL) Bottle, 600 mg Suspension NDC 0037-0442-67 - 8 fl oz (237 mL) Felbatol® (felbamate) Oral Suspension - Each 5 mL contains - 600 mg felbamate - Rx Only - SHAKE WELL - MEDA - PHARMACEUTICALS® LB-024F5-12 ...
-
INGREDIENTS AND APPEARANCEProduct Information